Zebrafish as screening model for detecting toxicity and drugs efficacy
Abstract
Embryonic and larval Danio rerio (zebrafish) is increasingly used as a toxicological model to conduct rapid in vivo tests and developmental toxicity assays; the zebrafish features high genetic homology to mammals, robust, phenotypes, high-throughput genetic and chemical screening have made it a powerful tool to evaluate in vivo toxicity. New methodologies of genome editing as CRISPR/Cas9; ZFN and TALEN make it a suitable model to perform studies to pair human genetic diseases as well. This review surveys recent studies employing zebrafish as experimental model, comparing it with other in vivo and in vitro models, presenting zebrafish as a potent vertebrate tool to evaluate drug toxicity and efficacy in order to facilitate more extensive, easy and comprehensive knowledge of new generation drugs.
Keywords
Introduction
Initially, in the early 1970s, the interest on zebrafish was as a model system, when it was selected to develop the first vertebrate assay enabling forward genetic screening[1]. During the subsequent 30 years, zebrafish was almost used to study organ development. This resulted in the characterization of an exceptionally large number of genes involved in vertebrate pathways, which contributed to the establishment of zebrafish as a relevant model for human disease and pharmaceutical researches[2]. For pharmacology investigations an attractive feature of zebrafish assays is the prospective to use them in medium-to-high-throughput screening mode, because the zebrafish is a small (5 cm for an adult and 5 mm for 7 days post-fertilization (dpf) larvae) and robust fish that is easy to maintain thanks to their high fecundity.
Earlier zebrafish have been used for evaluating the toxicity of agrochemical agents[3] but more recently, their use for toxicity assessment of pharmaceutical compounds has been greatly increased[4]. In zebrafish larvae, an in vivo toxicology evaluation can be reached in a week; the shorter time frame required performing comparable mammalian assays. Toxicology studies often disclose effects that require further investigation to explain purposes that are expensive and time consuming. Screening technologies exist and are being further developed in zebrafish, which should provide very early details of potential off-target effects on the cardiac system as well as other functions such as effects on central nervous system, on the intestinal tract, auditory and visual functions, pro-convulsant potential and bone formation. Therefore, the zebrafish technology should be considered as a useful pre-filter to support selection of the safest lead candidates as early as possible in the drug discovery process.
In drug discovery, cardiotoxicity is one of the major concerns for pharmaceutical companies, being a common, unfavorable complication associated with drugs used in oncological[5], neurological[6] or other treatments[7]. This fact has become an important issue, with particular relevance for children and adolescents, as they may be more susceptible to toxic effects, and often use these treatments off-label[8].
Many drugs (belonging to different chemical and pharmacological groups) can affect ionic channels, associated with the potential for QT interval prolongation in the heart’s electric cycle, leading to ionic channel blockade in the cardiomyocyte membrane[7]. Those events are linked to a higher risk of torsade de pointes (TdP) being a very complex process to accurately predict its scale. This fact is one of the most important outcomes of cardiotoxicity assessment of new molecules[9], together with the reduction in human ether-a-go-go-related gene (hERG), the alpha subunit of potassium ion channel that mediates the repolarizing current, an effect that can evolve into life-threatening pro-arrhythmic episodes. These undesirable side effects of non-antiarrhythmic compounds have prompted the withdrawal of several blockbuster drugs from the market[10,11], making necessary studies on mechanisms of hERG channel inhibition, providing significant insights into the molecular factors that determine state-, voltage-, and use-dependency of hERG current Block[11]. Some authors correlate also the increased expression of neuronal sodium channels within the heart to epilepsy-related cardiac arrhythmias associated with QT prolongation on the electrocardiogram[12]. As a result, a better understanding of channels as hERG and neuronal sodium channels, could improve treatments that develop side effects on cardiac repolarization. Using zebrafish embryos, Langheinrich et al.[13] reported that embryos expressing an orthologue hERG (named zERG), were affected by a range of QT-prolonging drugs, inducing severe arrhythmia. In vivo studies represent an essential step in drug development and toxicity studies, as current requirements are high and include in vivo and in vitro assays to increase drug efficacy, minimizing toxicity[13].
Despite higher animals have been for many year models of excellence used to evaluate drugs toxicity, the zebrafish presents itself as a reliable vertebrate model to determine, developmental toxicity, general toxicity and to perform an initial drug screening. Derived from its use, have been reported comparable results to the data obtained with higher models[14-16].
This manuscript surveys recent studies testing the cardiotoxicity of drugs used to treat pathologies in different animal models, identifying as early as feasibly possible potential safety liabilities of drugs selected for human evaluation[17].
Zebrafish as cardiotoxicological tool
Although physiological differences are evident between zebrafish and mammalian heart, the zebrafish has become a good option to study heart development[18] and heart regeneration[19]. The zebrafish has contributed to obtain measurements as action potential trough voltage mapping, to determine cells coupling[20], and this fact together with calcium signaling, are important for cardiomyocyte proliferation and differentiation[21].
The last decade’s large animals, such as mice, rats and rabbits, have been widely used to study cardiotoxicity after drug administration[22-24], presenting some limitations. For instance, rodents can be insensitive to compounds’ cardiotoxicity, particularly when the endpoint measurement is left ventricular contractile function[25]. This may be due to rodents’ ability to compensate loss of myocytes by recruiting alternative mechanisms.
According to the United States government, rodent and rabbit toxicity testing has been the standard for assessing acute toxicity since the 1950s. However, the process is costly and time consuming, which has led to a backlog in chemical testing[26]. Because of these limitations, the need for use of other alternative animal models has increased.
The zebrafish is particularly suitable for this purpose because it represents a vertebrate species, its genome has been sequenced[2], and a large number of synchronously developing, transparent embryos can be produced[11]. In particular, the zebrafish has a high cost-effect benefit and has become an important tool to evaluate Geno-cardiotoxicity, to study embryo development and general toxicity[4,10,11]. For instance, several compound screens, including some evaluating drug-induced cardiotoxicity and others already in preclinical trials, have successfully tested drug effects in zebrafish[27-29].
Although the zebrafish heart is two-chambered, its fundamental electrical properties are remarkably similar to those of humans. Zebrafish heart rate and action potential are analogous to those of humans[30,31]; also it presents highlighted genetics and regulatory networks similarities driving cell fate parallel those of higher vertebrates[31-33]. Moreover, cardiac performance in adult zebrafish can be detected by new noninvasive methods. It can be assessed by advancing conventional echocardiography with speckle-tracking analyses and changes in cardiac performance, and enables highly sensitive assessment of regional myocardial motion and deformation in high spatio-temporal resolution[34].
Then in vivo studies represent an essential step in drug development and toxicity study, and the zebrafish cardiotoxicity test has been reported very reliable, describing the potential toxicity of drugs to the human cardiovascular system[35].
Detection of doxorubicin toxicity using different animal models
Doxorubicin (most used trade name, adriamycin) is a potent anti-tumoral agent utilized as an important, broad anti-cancer drug to treat leukemia, lymphoma, breast cancer and small cell carcinoma of the lung[36-39]. The ability of doxorubicin to kill rapidly dividing cells and, in turn, slow disease progression has been acknowledged for over 30 years[40,41]. The introduction of this antineoplastic antibiotic is one of the major successes in oncology[36-39]. Though, in spite of the pharmacological advantage associated with its use, doxorubicin presents toxicological effects on noncancerous cells as well, leading to cardiotoxicity and recalcitrant heart failure at high cumulative doses[41]. This damage can manifest itself as arrhythmia, arterial hypertension, thromboembolism, angina pectoris, myocardial infarction, or heart failure[42]. For instance, a dose of anthracycline-doxorubicin of 500 mg/m2 of body surface area causes cardiac complications in 4%-36% of the treated patients[43]. Thus, understanding the mechanism which doxorubicin induces cardiac injury is crucial not only to avoid its cardiotoxic effect but also to improve the therapeutic use of doxorubicin.
Several reports highlight the cardiotoxicity of doxorubicin in different animal models [Table 1], focusing on children safety, where its pharmacokinetics has been assessed. The evaluation whether an age dependency in the clearance (CL) of doxorubicin exists has led to the conclusion that the lower CL in younger population should be considered, together with pharmacodynamics. Those results are especially important in cardiotoxicity, being essential to select the future dose for a protocol[44]. This issue has been addressed by using juvenile mice[45], concluding that treatment with high cumulative doses of doxorubicin induced cardiomyocyte atrophy, myofiber disarray, low levels of cardiomyocyte apoptosis, and altered expression of structural and regulatory proteins, normalization from the treatment was observed after a 13-week recovery period. Mostly, the studies of doxorubicin-induced cardiotoxicity perform a single injection followed by evaluation within one week[46-48].
Existing studies on DOXO cardiotoxicity
| Model | DOXO study | References |
|---|---|---|
| Juvenile mice | 5 weeks DOXO administration determines a decline in cardiac systolic function with cardiomyocytes atrophy, myofiber disarray, low levels of cardiomyocyte apoptosis, and altered expression of structural and regulatory proteins. 13-week recoveries period bring back to normality | [45] |
| Mice | Meloxicam abrogates the cardio toxic effect of DOXO in mice | [50] |
| Mice | Resveratrol generates cardiovascular protective effects by a heme oxygenase-1-mediated mechanism | [51] |
| Zebrafish (embryo-larva) | High DOXO doses had lethal effects; low DOXO doses resulted in sub-lethal effects, malformations, and changes of heart rate | [52] |
| Rats | DOXO genotoxicity evaluation. Enzyme-modified comet assay reported a significant induction of DNA damage in heart tissue | [58] |
Doxo has being studied in combination with protective compounds[49], the authors reported the effects of protective molecules and studied the underlying cardiotoxicity mechanism of doxorubicin. It was also reported that two different doses of meloxicam present a potential cardio-protective effect[50], and a combination of resveratrol/doxorubicin in mice was able to generate cardiovascular protective effects by a heme oxygenase-1 (HO-1)-mediated mechanism[51].
Beside the use of mice to evaluate cardiotoxical effect after drugs treatment, the zebrafish is presented as a costless vertebrate model with reduced complexity, promising to be a powerful tool to evaluate cardiotoxicity. It was evaluated lethal and sub-lethal doses of doxorubicin in embryo-larva at different time points, 4 and 120 h post fertilization (hpf)[52]. Higher doxorubicin doses had lethal effects, whereas lower concentrations resulted in sub-lethal effects and malformations, as well as changes in the heart rate[52]. Exploiting the transparency of the embryo has permitted detailed optical mapping and the characterization of the cardiac conduction system[53]. Heart rate measurement is quite easy in zebrafish, making it an attractive screening tool for assessing cardiovascular risk after treatment[54]. Most importantly, zebrafish can survive in the absence of cardiac output and in the presence of major vascular defects for several days, unlike many larger animals[55]. Collectively, these characteristics have made Danio rerio increasingly popular to test cardiotoxicity and cardiovascular developmental effects after drug administration as doxorubicin.
Atipsychotics toxicity testing
Antipsychotics are a class of medications primarily used to manage psychosis (including delusions, hallucinations, or disordered thought), particularly in schizophrenia and bipolar disorders, by alleviating such symptoms as hallucinations, both visual and auditory, and paranoid thoughts[56]. However, the first generation of antipsychotics has usually been associated with elevated cardiovascular mortality due to QTc interval prolongation and may cause TdP. Many anti-psychotic drugs had to be withdrawn from the market, and starting from 2005, the ICH E14 guidance has recommended conducting a “thorough QT/QTc study” aimed at assessing whether the drug has an effect on QT interval[57-59]. Early antipsychotic medications had important side effects, leading researchers to continue their work for better drugs, avoiding effects as severe ventricular arrhythmias and sudden cardiac death. The dysfunction of the cardioregulatory system may also be associated with functional and medication-related mechanisms rather than structural changes[60].
Some studies have assessed the cardiotoxicity of certain antipsychotics in mammalian models [Table 2]. For instance, clozapine has been found to induce myocarditis in rats, which exhibited inflammatory response, myocyte vacuolar degradation, myofiber necrosis and interstitial fibrosis[61,62]. Similarly, a cardiotoxic effect of clozapine in mice has been reported, detecting myocarditis, as well as inflammatory lesions after 7 or 14 days with 5, 10 or 25 mg/kg dose daily treatment.
Existing studies, describing cardiotoxicity of antipsychotic drugs
| Model | Antipsychotic study | Reference |
|---|---|---|
| Rats | Clozapine induces myocarditis, showing inflammatory respond, myocyte vacuolar degradation and myofiber necrosis | [61] |
| Mice | 7 or 14 days clozapine daily treatment causes myocarditis as well as inflammatory lesions after | [62] |
| Rats and mice | Histological determination of cardiotoxicological effect of antipsychotic as aripiprazole, olanzapine, quetiapine, risperidone or ziprasidone | [62] |
| Zebrafish larvae | Cardiotoxicological effects of first generation antipsychotics (aripiprazole, clozapine, olanzapine, quetiapine, risperidone and ziprasidone)on heart rate, morphology and motility | [65] |
In spite of these findings, there is a lack of systematic evidence of the cardiotoxicological effects of many antipsychotic drugs. On the other side, the cardiotoxicity of antipsychotics such as aripiprazole, olanzapine, quetiapine, risperidone and ziprasidone has not been investigated in rats, likely due to the high cost that such experiments would entail. As a result, most studies about the cadiotoxicity of these drugs in rats have relied on histological determination, which yields a poor understanding of their cardiotoxological effects[63,64]. This underlines the need for alternative, more economical models for these experiments.
The zebrafish emerges as a highly amenable model for toxicity studies of antipsychotics precisely for these reasons. Although mammalian toxicity studies remain the gold standard for risk assessment, the zebrafish has become a valid model due to the toxic responses that appear to be well conserved between mammalians and zebrafish[64]. Cardiotoxicological effects of aripiprazole, clozapine, olanzapine, quetiapine, risperidone and ziprasidone were documented[56].
Aripiprazole, clozapine, olanzapine, quetiapine, risperidone and ziprasidone (first-generation antipsychotics) were assessed using the zebrafish larvae. Different endpoints as the heart rate, edema presence, heart morphology, body shape, motility and the heart beat rate, were determined as cardiovascular toxicity endpoints. The authors concluded that the zebrafish model facilitates determination of the heart beat rate, and could thus be an attractive tool for cardiovascular risk assessment of atypical drugs to understand the variations in response to QT-prolonging drugs[65].
Thus, the use of the zebrafish as a model in these studies will facilitate more extensive, easy and comprehensive knowledge of drugs cardiotoxicity, generating a deeper understanding of that process. Similarly, the zebrafish is also an attractive screening tool for cardiovascular risk assessment after treatment with atypical antipsychotic drugs, as it facilitates the evaluation of the heart beat rate[66].
An animal model to evaluate genotoxicity
An important component in toxicology and drug development is to assess genotoxicity. This important issue has been evaluated through toxicological assays as, Ames test, comet, or in vitro and in vivo micronucleus assays; in the past few years, zebrafish has started to be considered as an in vivo alternative method to evaluate genotoxicity.
During years, rats have been extensively utilized to evaluate the genotoxicity of drugs through comet assay, micronucleus test and gene profiling techniques. Some studies using rats peripheral blood, shown a significant induction of DNA damage in heart tissue after doxorubicin treatment using the enzyme-modified comet assay[67].
Nevertheless, in the last few years, zebrafish has emerged as a relevant genotoxic tool. Adult zebrafish were exposure to a 2-week treatment of the alkylating agent methyl methanesulfonate. The comet assay was employed to evaluate the presence of micronuclei in gonad, liver, or gild[68,69]. On the basis of this study, zebrafish can be considered an efficient vertebrate model to study genotoxicity through comet assay and the micronucleus test. They argued that this model proved appropriate for the detection of genotoxicity in primary male and female gonad cells as well as using histological sections of the gonads from zebrafish, respectively[68].
Finally noteworthy, PAC2 zebrafish cell line has been used as in vitro model to evaluate genotoxicity. Cells were exposed to a short-term (2 h) using a concentration range. The study reveals genotoxic pressure by genotoxic agents. As a note, this cell line compared to another fish cell line (PLHC-1, trout hepatocytes), showed less sensitivity upon short-term exposure to the genotoxicants tested[61-63]. Thus, those reports bring to light zebrafish as in vivo and in vitro model to study genotoxicity[68-72].
Cardiotoxicity of small molecules: drug screening using the fish
Cardiovascular toxicity is a major limiting factor in drug development and requires multiple cost-effective models to perform toxicological evaluation[73,74]. Zebrafish is now a well-validated animal model to study treatment with small molecules, as well as to elucidate biological functions, and deciphering the mechanism of bioactive compounds[74]. The model has emerged as a powerful system for small molecule screening and for novel biological and therapeutic discoveries[74]. For instance through in situ hybridization may be reported the expression of some target genes[75]. That assay requires prior the knowledge of the biologic process and depends on the selected molecular target, which should be critical for the developmental events[75].
In an attempt to evaluate toxicity of medicaments and other chemicals, new methodologies focusing on cardiomyocyte properties or computational models are under development[76,77]. Despite their usefulness, these strategies do not provide enough information about toxicity in the organism as an effect of the secondary metabolism derived from drugs, or the drug effects in other cellular lines present in the heart[76-79].
Primary cardiomyocytes derived from human embryonic stem cells[78] are used to evaluate cardiotoxicity; however the general consensus is that a reliable in vivo model is needed. Additionally some in silico approaches (computational techniques) can assess the ionic flow by the action of a drug, reaching some results about Na+, K+, L-type Ca2+ channels or multiple membrane ion channels in cardiomyocytes. These computational models show up as cardiotoxicological method to evaluate the actions of drugs on cardiac electrical activities at cellular and tissue[79].
Cardiac electrophysiology and modeling drug-channel studies, have documented many improvements in the last decades, although, the generation of a virtual heart model for drug safety assessment is still a major challenge. Firstly, more studies are necessary to develop biophysically accurate models: the zebrafish could constitute a good approach for drug-channel interactions and cardiac electrophysiology[80]. Although simpler than humans, zebrafish are also complex vertebrates that maintain equally elaborate mechanisms to activate or relieve the effects of exogenous chemicals[81].
Thus, the close resemblance of the genetic cascade governing heart development in zebrafish to that of humans has propelled the zebrafish system as a cost-effective model to conduct pharmacological screens on developing embryos and larvae as well as to provide data to generate computational models to evaluate in silico drugs studies[79,81].
Modeling human diseases using the zebrafish
Understanding syndromes with genetic bases has become an important topic in medicine, with the hope that new treatments will be discovered. The zebrafish appears as a fast model to study de novo mutations and genetic diseases. Using genomic editing approaches as CRISPR/Cas9[82], or artificial site-specific nucleases such as zinc-finger nucleases, and transcription activator-like nucleases[83,84], genes can be inactivated in vivo, mimicking human phenotypes, and obtaining information about human diseases with genetic background[81,85,86].
A genetic syndrome called Dravet syndrome (DS), is linked to more than 300 de novo mutations present in a neuronal voltage-gated sodium channel. It was reported to have screened a chemical library, constituted of around 1000 compounds[87]. From this study, the authors identified four compounds with the ability to rescue the behavioral seizure component and reported that dimethadione suppressed associated electrographic seizure activity. The authors used a mutant zebrafish line called scn1lab DS to reach this conclusion. The importance to study this syndrome is its association with a higher risk of sudden death in children[87,88].
Similarly to the DS syndrome, other genetic modifications that lead to heart disorders associated with structural heart defects can be found, including the human-like cardiomyopathies (DCMs)[89], which together with the silent heart or the pickwick mutans, present poor heart contractility. DCMs are characterized by ventricle and/or atrium enlargement. DCM syndrome has been studied with two particular mutant zebrafish lines: tnnt2 and laminin α-4 integrin linked kinase. In both lines, endothelial cells and cardiomyocytes were affected, in a similar manner similar to familial phenotype presented with DCM in human[90].
In a similar manner other authors showed the ability of zebrafish to simulate amyloid light-chain amyloidosis, a plasma cell disorder that causes rapidly progressive cardiomyopathy. Protein injection into the blood stream of zebrafish embryos led to a severe cardiomyopathy; showing a clear cardiac dysfunction, cell death and pericardial edema[81].
Thus, the use of this model is uniquely positioned among vertebrates as a platform for small molecule screening and efficacy testing: the zebrafish is used to identify novel drugs associated with molecular pathways, with the purpose to treat human diseases. The use of genetic manipulation in zebrafish is an efficient way to assess the roles of individual genes in disease processes. As such, it represents a route to the identification of novel drugs[91]. In addition, zebrafish can be used to provide insight into the biological function of the many candidate genes being rapidly identified in human genome[92].
Zebrafish as tool to evaluate hepatotoxicity
Toxicology studies are needed to determine the suitability and consequences of drug administration in humans. In the process of drug discovery, one of the main concerns is to evaluate drug hepatotoxicity, which is assessed using preclinical cell culture, animal models and clinical trials. However, drug hepatotoxicity is difficult to detect prior to human use, limiting the discovery and development of novel therapies using conventional models[93,94].
As a result of this fact, to develop new in vivo and in vitro models for efficacy and safety testing is needed. Therefore, to set up better tools to screen for drug-induced liver injury (DILI) of large compound libraries in early stages of drug development will allowed to gain a better understanding of hepatotoxicity. Being this fact the most common cause of drug withdrawal[95].
Zebrafish liver organogenesis starts at 3 days post fertilization (dpf) and is fully functional by 5 dpf[96]. The tri-lobed liver of the zebrafish is similar to that of the mammal with regard to biological function, including the processing of lipids, vitamins, proteins and carbohydrates, as well as the synthesis of serum proteins[97]. Some studies suggest that drugs are metabolized when exposed to zebrafish embryos by similar reactions to those in humans[98,99].
Zebrafish possess a wide range of cytochrome P450 enzymes that allow metabolic reactions including hydroxylation, conjugation, oxidation, demethylation and de-ethylation[99]. Following exposure to a range of hepatotoxic drugs, the zebrafish liver develops histological patterns of injury comparable to those of mammalian liver, and biomarkers for liver injury can be quantified in the zebrafish circulation[99].
Since hepatotoxicity is derived from metabolic processes, zebrafish are useful to study DILI with in vivo models. Parameters such as apoptosis, liver opacity or size, can be evaluated in the zebrafish. The availability of specific transgenic lines labeling the liver, such as fabp10:RFP, allows liver damage visualization after the treatment. Analysis of fluorescent intensity can be informative with regard to size or the number of hepatocytes[100].
However, has been described the inconvenient to work with the larvae, arguing that the CYP system, which plays an essential role in drug metabolism, is not yet fully developed in larvae and suggesting that some CYPs appear to be lacking in the early zebrafish life[101]. Furthermore, zebrafish embryos and larvae showed no or low biotransformation capacity of four human CYP-specific substrates, dextromethorphan, diclofenac, testosterone and midazolam[101]. In contrast, has been reported the larva as a promising tool capable of distinguishing between hepatotoxic and non-hepatotoxic chemical analogues, implying that it may be applied as a screening model for DILI[102].
Also a recent study has developed a new experimental procedure (ZeGlobalTox assay) that addresses the organ-specific toxicity of different drugs on zebrafish larvae (up to 5 dpf). It permits the independent analysis of cardio-, neuro-, and hepatotoxicity effects in the same animal. The main concern was that drug-induced teratogenicity (developmental toxicity) and/or mortality could mask possible organ-toxicities appearing later in development[103].
Zebrafish provide a complex, in vivo and functional vertebrate system to evaluate DILI. A deeper understanding of the zebrafish model of liver toxicity, which has been underutilized, will therefore permit better drug prediction and reduce the need for drug withdrawal.
Advantages of the zebrafish model with respect to cost and time
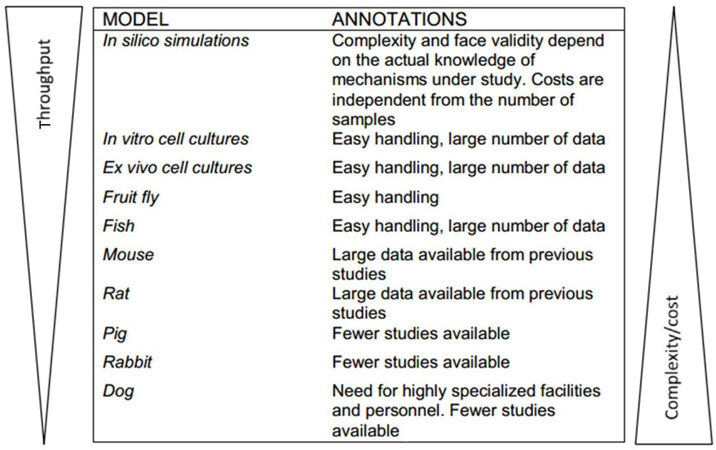
As it has been described, toxicity testing of drugs in recent years has employed various animal models[104,105]. Although mice, rats, rabbits and dogs are excellent models according to most standards, they present some serious limitations [Figure 1].
Figure 1. Representation of different biological system and animal model used to test cardiotoxicity drugs, classified in function of face validity, costs and throughput efficiency
Experiments evaluating drug toxicity typically requires large numbers of animals increasing the monetary cost of the experiments significantly as well as, those animals handling is often quite time-consuming. The small size of zebrafish renders them, ideal for experiments, being more easily handled and being associated with lower costs [Figure 1], and providing researchers and those concerned with animal welfare, with an alternative to work according to the 3Rs principles (refinement, reduction and replacement)[106].
As a toxicology model, zebrafish has the potential to reveal the pathways of developmental toxicity due to their similarity with those of mammals. Zebrafish therefore, provides a sound basis for the risk assessment of drug administration in humans. Thus, in many respects, the use of the zebrafish as a model for studies of cardio- behavior, genotoxicity or hepatotoxicity would allow the researcher to overcome many of the challenges presented by using other animals models, including limitations on sample size and higher monetary and time costs[66,69,74,82].
Conclusion
Increases of studies evaluating drug’s toxicity upon animal have been reported[104,105]. While larger animals such as mice, rats, rabbits and dogs are generally appropriate models to use; they present significant limitations, particularly with respect to cost, time, ethical concerns and sample size. On the other hand, in vitro tests used to assess biosafety lack the potency and the translational attributes of a whole animal.
The zebrafish is a good alternative for biosafety studies due to its small size, genetics background, higher breeding capabilities, and most importantly, due the similarities of its molecular pathways and physiology with that of humans. The emergence of zebrafish as a model for assessing cardio- neuro- or geno- toxicity of drugs is reflective of its advantages over other animal models with respect to the principles of the 3Rs (replacement, reduction and refinement). On the other hand, the ease of genome editing, using new mutagenesis techniques such as CRISPR/Cas9[79] (in the fish, will make suitable future studies to pair human genetic mutations with their molecular functions.
Zebrafish are amenable small teleost, incessantly used for drug screening, efficacy studies and toxicity testing[65,68,69,86]. Although cardiotoxicity, behavior alteration or teratogenecity determinations can be determined using zebrafish, to evaluate hepatotoxicity derived from new generation drugs treatments is still under testing. In light of these advantages, we emphasize the zebrafish model as an excellent vertebrate toxicological model with potential to contribute to significantly improve drug development in toxicology.
In conclusion, the zebrafish presents a powerful in vivo preclinical model for assessing the adverse effects of a wide range of drugs as well as to determine drug efficacy. Its use, in conjunction with approaches based on those presented in this review, would contribute significantly to the literature and would facilitate the implementation of innovative, comprehensive, and cost-effective testing strategies.
Declarartions
AcknowledgmentsThe authors thank Levent Altinoglu for his assistance in the manuscript writing and Professor Annarosa Leri for her support.
Authors’ contributionsDesign, literature research, drafting, critical revision of contents and final approval of the manuscript: Caballero MV, Candiracci M
Financial support and sponsorshipNone.
Conflicts of interestBBD-Biophenix Biobide employed Maria Virginia Caballero during the writing paper.
Patient consentNot applicable.
Ethics approvalNot applicable.
Copyright© The Author(s) 2018.
REFERENCES
1. Grunwald DJ, Eisen JS. Headwaters of the zebrafish emergence of a new model vertebrate. Nat Rev Genet 2002;3:717-24.
2. Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assunção JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Threadgold G, Harden G, Ware D, Begum S, Mortimore B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Lloyd C, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Ürün Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberländer M, Rudolph-Geiger S, Teucke M, Lanz C, Raddatz G, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Schuster SC, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nüsslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J, Stemple DL. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013;496:498-503.
3. Bretaud S, Lee S, Guo S. Sensitivity of zebrafish to environmental toxins implicated in Parkinson's disease. Neurotoxicol Teratol 2004;26:857-64.
4. Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci 2005;86:6-19.
5. Hong RA, Iimura T, Sumida KN, Eager RM. Cardio-oncology/onco-cardiology. Clin Cardiol 2010;12:733-7.
6. Schug SA, Saunders D, Kurowski I, Paech MJ. Neuraxial drug administration: a review of treatment options for anaesthesia and analgesia. CNS Drugs 2006;20:917-33.
7. Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, Siegl PK, Strang I, Sullivan AT, Wallis R, Camm AJ, Hammond TG. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res 2003;58:32-45.
8. Ramjaun A, AlDuhaiby E, Ahmed S, Wang L, Yu E, Nathan PC, Hodgson DC. Echocardiographic detection of cardiac dysfunction in childhood cancer survivors: how long is screening required? Pediatr Blood Cancer 2015;62:2197-203.
9. Fradley MG, Moslehi J. QT prolongation and oncology drug development. Card Electrophysiol Clin 2015;7:341-55.
10. Shah RR. Pharmacogenetic aspects of drug-induced torsade de pointes: potential tool for improving clinical drug development and prescribing. Drug Saf 2004;27:145-72.
11. Thomas D, Karle CA, Kiehn J. The cardiac hERG/IKr potassium channel as pharmacological target: structure, function, regulation, and clinical applications. Curr Pharm Des 2006;12:2271-83.
12. Biet M, Morin N, Lessard-Beaudoin M, Graham RK, Duss S, Gagné J, Sanon NT, Carmant L, Dumaine R. Prolongation of action potential duration and QT interval during epilepsy linked to increased contribution of neuronal sodium channels to cardiac late Na+ current: a potential mechanism for sudden death in epilepsy. Circ Arrhythm Electrophysiol 2015;8:912-20.
13. Langheinrich U, Vacun G, Wagner T. Zebrafish embryos express an orthologue of HERG and are sensitive toward a range of QT-prolonging drugs inducing severe arrhythmia. Toxicol Appl Pharmacol 2003;193:370-82.
14. Ducharme NA, Reif DM, Gustafsson JA, Bondesson M. Comparison of toxicity values across zebrafish early life stages and mammalian studies: implications for chemical testing. Reprod Toxicol 2014;55:3-10.
15. Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet 2007;85:353-67.
16. Wiley DS, Redfield SE, Zon LI. Chemical screening in zebrafish for novel biological and therapeutic discovery. Methods Cell Biol 2017;138:651-79.
17. Garcia GR, Noyes PD, Tanguay RL. Advancements in zebrafish applications for 21st century toxicology. Pharmacol Ther 2016;161:11-21.
18. Bartman T, Walsh EC, Wen KK, McKane M, Ren J, Alexander J, Rubenstein PA, Stainier D. Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol 2004;2:E129.
20. Panáková D, Werdich AA, Macrae CA. Wnt11 patterns a myocardial electrical gradient through regulation of the L-type Ca(2+) channel. Nature 2010;466:874-8.
21. Andersen ND, Ramachandran KV, Bao MM, Kirby ML, Pitt GS, Hutson MR. Calcium signaling regulates ventricular hypertrophy during development independent of contraction or blood flow. J Mol Cell Cardiol 2015;80:1-9.
22. Guenancia C, Hachet O, Aboutabl M, Li N, Rigal E, Cottin Y, Rochette L, Vergely C. Overweight in mice, induced by perinatal programming, exacerbates doxorubicin and trastuzumab cardiotoxicity. Cancer Chemother Pharmacol 2016;77:777-85.
23. Vasilaki F, Tsitsimpikou C, Tsarouhas K, Germanakis I, Tzardi M, Kavvalakis M, Ozcagli E, Kouretas D, Tsatsakis AM. Cardiotoxicity in rabbits after long-term nandrolone decanoate administration. Toxicol Lett 2016;241:143-51.
24. Lamore SD, Kamendi HW, Scott CW, Dragan YP, Peters MF. Cellular impedance assays for predictive preclinical drug screening of kinase inhibitor cardiovascular toxicity. Toxicol Sci 2013;135:402-13.
25. Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowki D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J, George S, Morgan JA, Harris DM, Ismail NS, Chen JH, Schoen FJ, Van den Abbeele AD, Demetri GD, Force T, Chen MH. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007;370:2011-9.
26. National Research Council. Toxicity testing in the 21st century: a vision and astrategy. Washington, DC: The National Academies Press; 2007.
27. Rennekamp AJ, Peterson RT. 15 years of zebrafish chemical screening. Curr Opin Chem Biol 2015;24C:58-70.
28. Fang M, Guo J, Chen D, Li A, Hinton DE, Dong W. Halogenated carbazoles induce cardiotoxicity in developing zebrafish embryos (Danio rerio). Environ Toxicol Chem 2016;35:2523-9.
29. Cui G, Chen H, Cui W, Guo X, Fang J, Liu A, Chen Y, Lee SM. FGF2 prevents sunitinib-induced cardiotoxicity in zebrafish and cardiomyoblast H9c2 cells. Cardiovasc Toxicol 2016;16:46-53.
30. Arnaout R, Ferrer T, Huisken J, Spitzer K, Stainier DY, Tristani-Firouzi M, Chi NC. Zebrafish model for human long QT syndrome. Proc Natl Acad Sci U S A 2007;104:11316-21.
31. Sedmera D, Reckova M, deAlmeida A, Sedmerova M, Biermann M, Volejnik J, Sarre A, Raddatz E, McCarthy RA, Gourdie RG, Thompson RP. Functional and morphological evidence for a ventricular conduction system in zebrafish and Xenopus hearts. Am J Physiol Heart Circ Physiol 2003;284:H1152-60.
32. Vacaru AM, Unlu G, Spitzner M, Mione M, Knapik EW, Sadler KC. In vivo cell biology in zebrafish - providing insights into vertebrate development and disease. J Cell Sci 2014;127:485-95.
34. Hein SJ, Lehmann LH, Kossack M, Juergensen L, Fuchs D, Katus HA, Hassel D. Advanced echocardiography in adult zebrafish reveals delayed recovery of heart function after myocardial cryoinjury. PLoS One 2015;10:e0122665.
35. Zhang CJ, Willett C, Fremgen T. Zebrafish: an animal model for toxicological studies. Curr Protoc Toxicol 2003;1:7.
36. Johnson S, Smith AG, Loffler H, Osby E, Juliusson G, Emmerich B, Wyld PJ, Hiddemann W. Multicentre prospective randomised trial of fludarabine versus cyclophosphamide, doxorubicin, and prednisone (CAP) for treatment of advanced-stage chronic lymphocytic leukaemia. The French Cooperative Group on CLL. Lancet 1996;347:1432-8.
37. Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, Ingle JN, Cooper MR, Hayes DF, Tkaczuk KH, Fleming G, Holland JF, Duggan DB, Carpenter JT, Frei E, Schilsky RL, Wood WC, Muss HB, Larry N. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer norton. J Clin Oncol 2003;21:976-83.
38. Peer D, Karp JM, Omid SH, Farokhzad C, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2007;2:751-60.
39. Woll PJ, Reichardt P, Cesne AL, Bonvalot S, Azzarelli A, Hoekstra HJ, Leahy M, Coevorden FV, Verweij J, Hogendoorn PCW, Ouali M, MSc Marreaud S, Bramwell VHC, Hohenberger P. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol 2012;13:1045-54.
40. Ling YH, el-Naggar AK, Priebe W, Perez-Soler R. Cell cycle-dependent cytotoxicity, G2/M phase arrest, and disruption of p34cdc2/cyclin B1 activity induced bydoxorubicin in synchronized P388 cells. Mol Pharmacol 1996;49:832-41.
41. Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol 2013;65:157-70.
42. Carbalho FS, Burgeiro A, Garcia R, Moreno AJ, Carbalho RA, Oliveira PJ. Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med Res Rev 2014;34:106-35.
43. Schlitt A, Jordan K, Vordermark D, Schwamborn J, Langer T, Thomssen C. Cardiotoxicity and oncological treatments. Dtsch Arztebl Int 2014;111:161-8.
44. Völler S, Boos J, Krischke M, Würthwein G, Kontny NE, Boddy AV, Hempel G. Age-dependent pharmacokinetics of doxorubicin in children with cancer. Clin Pharmacokinet 2015;54:1139-49.
45. Zhu W, Shou W, Payne RM, Caldwell R, Field LJ. A mouse model for juvenile doxorubicin-induced cardiac dysfunction. Pediatr Res 2008;64:488-94.
46. Kim KH, Oudit GY, Backx PH. Erythropoietin protects against doxorubicin induced cardiomyopathy via a phosphatidylinositol 3-kinase-dependet pathway. J Pharmacol Exp Ther 2008;324:160-9.
47. Fischer PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation 2005;111:1601-10.
48. Delgado RM III, Nawar MA, Zewail AM, Kar B, Vaughn WK, Wu KK, Aleksic N. Sivasubramanian N, McKay K, Mann DL, Willerson JT. Cyclooxygenase-2 inhibitor treatment improves left ventricular function and mortality in a murine model of doxorubicin-induced heart failure. Circulation 2004;109:1428-33.
49. Simunek T, Striba M, Popelova O, Adamcova M, Hrdina R, Gers V. Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep 2009;61:154-71.
50. Hassan MH, El-Beshbishy HA, Aly H, Attia SM, Bahashwan SA, Ghobara MM. Modulatory effects of meloxicam on cardiotoxicity and antitumor activity of doxorubicin in mice. Cancer Chemother Pharmacol 2014;74:559-69.
51. Gu J, Song ZP, Gui DM, Hu W, Chen YG, Zhang DD. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in lymphoma nude mice by heme oxygenase-1 induction. Cardiovasc Toxicol 2012;12:341-9.
52. Chang C, Wu SL, Zhao XD, Zhao CT, Li YH. Developmental toxicity of doxorubicin hydrochloride in embryo-larval stages of zebrafish. Biomed Mater Eng 2014;24:909-16.
53. Chi NC, Shaw RM, Jungblut B, Huisken J, Ferrer T, Arnaout R, Scott I, Beis D, Xiao T, Baier H, Jan LY, Tristani-Firouzi M, Stainier DY. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biol 2008;6:e109.
54. Musso G, Tasan M, Mosimann C, Beaver JE, Plovie E, Carr LA, Chua HN, Dunham J, Zuberi K, Rodriguez H, Morris Q, Zon L, Roth FP, MacRae CA. Novel cardiovascular gene functions revealed via systematic phenotype prediction in zebrafish. Development 2014;141:224-35.
55. Rocke J, Lees J, Packham I, Chico T. The zebrafish as a novel tool for cardiovascular drug discovery. Recent Pat Cardiovasc Drug Discov 2009;4:1-5.
56. Leon C, Gerretsen P, Uchida H, Suzuki T, Rajji T, Mamo DC. Sensitivity to antipsychotic drugs in older adults. Curr Psychiatry Rep 2010;12:28-33.
57. Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH. Timing of new black box warnings and withdrawals for prescription medications. J Am Med Assoc 2002;287:2215-20.
58. Shah RR. Can pharmacogenetics help rescue drugs withdrawn from the market? Pharmacogenomics 2006;7:889-908.
59. Stockbridge N, Morganroth J, Shah RR, Garnett C. Dealing with global safety issues: was the response to QT-liability of non-cardiac drugs well coordinated? Drug Saf 2013;36:167-82.
60. Koponen H, Alaräisänen A, Saari K, Pelkonen O, Huikuri H, Raatikainen MJ, Savolainen M, Isohanni M. Schizophrenia and sudden cardiac death. Nord J Psychiatry 2008;62:342-5.
61. Abdel-Wahab BA, Metwally ME, El-khawanki MM, Hashim AM. Protective effect of captopril against clozapine-induced myocarditis in rats: role of oxidative stress, proinflammatory cytokines and DNA damage. Chem Biol Interact 2014;216:43-52.
62. Wang JF, Min JY, Hampton TG, Amende I, Yan X, Malek S, Abelmann WH, Green AI, Zeind J, Morgan JP. Clozapine-induced myocarditis: role of catecholamines in a murine model. Eur J Pharmacol 2008;592:123-7.
63. Dang R, Guo Y, Cai H, Yang R, Liang D, Lv C, Jiang P. Effects of prolonged antipsychotic administration on neuregulin-1/ErbB signaling in rat prefrontal cortex and myocardium: implications for the therapeutic action and cardiac adverse effect. J Toxicol Sci 2016;41:303-9.
64. Barbazuk WB, Korf I, Kadavi C, Heyen J, Tate S, Wun E, Bedell JA, McPherson JD, Johnson SL. The syntenic relationship of the zebrafish and human genomes. Genome Res 2000;10:1351-8.
65. Lee SH, Kim HR, Han RX, Oqani RK, Jin DI. Cardiovascular risk assessment of atypical antipsychotic drugs in a zebrafish model. J Appl Toxicol 2013;33:466-70.
66. Pylatiuk C, Sanchez D, Mikut R, Alshut R, Reischl M, Hirth S, Rottbauer W, Just S. Automatic zebrafish heartbeat detection and analysis for zebrafish embryos. Zebrafish 2014;11:379-83.
67. Manjanatha MG, Bishop ME, Pearce MG, Kulkarni R, Lyn-Cook LE, Ding W. Genotoxicity of doxorubicin in F344 rats by combining the comet assay, flow-cytometric peripheral blood micronucleus test, and pathway-focused gene expression profiling. Environ Mol Mutagen 2014;55:24-34.
68. Faßbender C, Braunbeck T. Assessment of genotoxicity in gonads, liver and gills of zebrafish (Danio rerio) by use of the comet assay and micronucleus test after in vivo exposure to methyl methanesulfonate. Bull Environ Contam Toxicol 2013;91:89-95.
69. Kovács R, Csenki Z, Bakos K, Urbányi B, Horváth Á, Garaj-Vrhovac V, Gajski G, Gerić M, Negreira N, López de Alda M, Barceló D, Heath E, Kosjek T, Žegura B, Novak M, Zajc I, Baebler Š, Rotter A, Ramšak Ž, Filipič M. Assessment of toxicity and genotoxicity of low doses of 5-fluorouracil in zebrafish (Danio rerio) two-generation study. Water Res 2015;77:201-12.
70. Devaux A, Pesonen M, Monod G. Alkaline comet assay in rainbow trout hepatocytes. Toxicol In Vitro 1997;11:71-9.
71. Šrut M, Traven L, Štambuk A, Kralj S, Žaja R, Mićović V, Klobučar GIV. Genotoxicity of marine sediments in the fish hepatoma cell line PLHC-1 as assessed by the Comet assay. Toxicol In Vitro 2011;25:308-14.
72. Šrut M, Bourdineaud JP, Štambuk A, Klobučar GI. Genomic and gene expression responses to genotoxic stress in PAC2 zebrafish embryonic cell line. J Appl Toxicol 2015;35:1381-9.
73. Mladěnka P, Applová L, Patočka J, Costa VM, Remiao F, Pourová J, Mladěnka A, Karlíčková J, Jahodář L, Vopršalová M, Varner KJ, Štěrba M; TOX-OER and CARDIOTOX Hradec Králové Researchers and Collaborators. Comprehensive review of cardiovascular toxicity of drugs and related agents. Med Res Rev 2018; doi: 10.1002/med.21476.
74. Chan J, Bayliss PE, Wood JM, Roberts TM. Dissection of angiogenic signaling in zebrafish using a chemical genetic approach. Cancer Cell 2002;1:257-67.
75. Jing L, Durand EM, Ezzio C, Pagliuca SM, Zon LI. In situ hybridization assay-based small molecule screening in zebrafish. Curr Protoc Chem Biol 2012;4:110236.
76. Lancaster MC, Sobie EA. Improved prediction of drug-induced Torsades de Pointes through simulations of dynamics and machine learning algorithms. Clin Pharmacol Ther 2016;100:371-9.
77. Glinka A, Polak S. The effects of six antipsychotic agents on QTc an attempt to mimic clinical trial through simulation including variability in the population. Comput Biol Med 2014;47:20-6.
78. Holmgren G, Synnergren J, Bogestål Y, Améen C, Åkesson K, Holmgren S, Lindahl A, Sartipy P. Identification of novel biomarkers for doxorubicin-induced toxicity in human cardiomyocytes derived from pluripotent stem cells. Toxicology 2015;328:102-11.
79. Yuan Y, Bai X, Luo C, Wang K, Zhang H. The virtual heart as a platform for screening drug cardiotoxicity. Br J Pharmacol 2014;172:5531-47.
80. Pott A, Rottbauer W, Just S. Functional genomics in zebrafish as a tool to identify novel antiarrhythmic targets. Curr Med Chem 2014;21:1320-9.
81. Mishra S, Guan J, Plovie E, Seldin DC, Connors LH, Merlini G, Falk RH, MacRae CA, Liao R. Human amyloidogenic light chain proteins result in cardiac dysfunction, cell death, and early mortality in zebrafish. Am J Physiol 2013;305:H95-103.
82. Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JR, Joung JK. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol 2013;31:227-31.
83. Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG, Tan W, Penheiter SG, Ma AC, Leung AY, Fahrenkrug SC, Carlson DF, Voytas DF, Clark KJ, Essner JJ, Ekker SC. In vivo genome editing using a high-efficiency TALEN system. Nature 2012;491:114-8.
84. Doyon Y, McCammon JM, Miller JC, Faraji F, Ngo C, Katibah GE, Amora R, Hocking TD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Amacher SL. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol 2008;26:702-8.
85. Marelli F, Persani L. How zebrafish research has helped in understanding thyroid diseases. F1000Res 2017;6:2137.
87. Dinday MT, Baraban SC. Large-scale phenotype-based antiepileptic drug screening in a zebrafish model of dravet syndrome. eNeuro 2015;2:ENEURO.0068-15.2015.
88. Dravet C, Bureau M, Oguni H, Fukuyama Y, Cokar O. Severe myoclonic epilepsy in infancy: Dravet syndrome. Adv Neurol 2005;95:71-102.
89. Ceulemans B, Boel M, Leyssens K, Van Rossem C, Neels P, Jorens PG, Lagae L. Successful use of fenfluramine as an add-on treatment for Dravet syndrome. Epilepsia 2012;53:1131-9.
90. Stainier DY, Fouquet B, Chen JN, Warren KS, Weinstein BM, Meiler SE, Mohideen MA, Neuhauss SC, Solnica-Krezel L, Schier AF, Zwartkruis F, Stemple DL, Malicki J, Driever W, Fishman MC. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development 1996;123:285-92.
91. Knöll R, Postel R, Wang J, Krätzner R, Hennecke G, Vacaru AM, Vakeel P, Schubert C, Murthy K, Rana BK, Kube D, Knöll G, Schäfer K, Hayashi T, Holm T, Kimura A, Schork N, Toliat MR, Nürnberg P, Schultheiss HP, Schaper W, Schaper J, Bos E, Den Hertog J, van Eeden FJ, Peters PJ, Hasenfuss G, Chien KR, Bakkers J. Laminin-alpha4 and integrin-linked kinase mutations cause human cardiomyopathy via simultaneous defects in cardiomyocytes and endothelial cells. Circulation 2007;116:515-25.
92. Kettleborough RN, Busch-Nentwich EM, Harvey SA, Dooley CM, de Bruijn E, van Eeden F, Sealy I, White RJ, Herd C, Nijman IJ, Fényes F, Mehroke S, Scahill C, Gibbons R, Wali N, Carruthers S, Hall A, Yen J, Cuppen E, Stemple DL. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 2013;496:494-7.
93. Zhu XW, Li SJ. In silico prediction of drug-induced liver injury based on adverse drug reaction reports. Toxicol Sci 2017;158:391-400.
94. Sewing S, Boess F, Moisan A, Bertinetti-Lapatki C, Minz T, Hedtjaern M, Tessier Y, Schuler F, Singer T, Roth AB. Establishment of a predictive in vitro assay for assessment of the hepatotoxic potential of oligonucleotide drugs. PLoS One 2016;11:e0159431.
95. Kleiner DE. Drug-induced liver injury: the hepatic pathologist's approach. Gastroenterol Clin North Am 2017;46:273-96.
96. Chu J, Kirsten C, Sadler A. New school in liver development: lessons from zebrafish. Hepatology 2009;50:1656-63.
97. Menke AL, Spitsbergen JM, Wolterbeek AP, Woutersen RA. Normal anatomy and histology of the adult zebrafish. Toxicol Pathol 2011;39:759-75.
98. Quinlivan VH, Farber SA. Lipid uptake, metabolism, and transport in the larval zebrafish. Front Endocrinol (Lausanne) 2017;8:319.
99. Vliegenthart AD, Tucker CS, Del Pozo J, Dear JW. Zebrafish as model organisms for studying drug-induced liver injury. Br J Clin Pharmacol 2014;78:1217-27.
100. Zhang X, Li C, Gong Z. Development of a convenient in vivo hepatotoxin assay using a transgenic zebrafish line with liver-specific dsred expression. PLoS One 2014;9:e91874.
101. Saad M, Matheeussen A, Bijttebier S, Verbueken E, Pype C, Casteleyn C, Van Ginneken C, Apers S, Maes L, Cos P, Van Cruchten S. In vitro CYP-mediated drug metabolism in the zebrafish (embryo) using human reference compounds. Toxicol In Vitro 2017;42:329-36.
102. Mesens N, Crawford AD, Menke A, Hung PD, Van Goethem F, Nuyts R, Hansen E, Wolterbeek A, Van Gompel J, De Witte P, Esguerra CV. Are zebrafish larvae suitable for assessing the hepatotoxicity potential of drug candidates? J Appl Toxicol 2015;35:1017-29.
103. Cornet C, Calzolari S, Miñana-Prieto R, Dyballa S, van Doornmalen E, Rutjes H, Savy T, D'Amico D, Terriente J. ZeGlobalTox: an innovative approach to address organ drug toxicity using zebrafish. Int J Mol Sci 2017;18:E864.
104. Nahar K, Gupta N, Gauvin R, Absar S, Patel B, Gupta V, Khademhosseini A, Ahsan F. In vitro, in vivo and ex vivo models for studying particle deposition and drug absorption of inhaled pharmaceuticals. Eur J Pharm Sci 2013;49:805-18.
105. Farghali H, Kgalalelo Kemelo M, Wojnarová L, Kutinová Canová N. In vitro and in vivo experimental hepatotoxic models in liver research: applications to the assessment of potential hepatoprotective drugs. Physiol Res 2016;65:S417-25.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Caballero MV, Candiracci M. Zebrafish as screening model for detecting toxicity and drugs efficacy. J Unexplored Med Data 2018;3:4. http://dx.doi.org/10.20517/2572-8180.2017.15
AMA Style
Caballero MV, Candiracci M. Zebrafish as screening model for detecting toxicity and drugs efficacy. Journal of Unexplored Medical Data. 2018; 3: 4. http://dx.doi.org/10.20517/2572-8180.2017.15
Chicago/Turabian Style
Caballero, Maria Virginia, Manila Candiracci. 2018. "Zebrafish as screening model for detecting toxicity and drugs efficacy" Journal of Unexplored Medical Data. 3: 4. http://dx.doi.org/10.20517/2572-8180.2017.15
ACS Style
Caballero, MV.; Candiracci M. Zebrafish as screening model for detecting toxicity and drugs efficacy. J. Unexplored. Med. Data. 2018, 3, 4. http://dx.doi.org/10.20517/2572-8180.2017.15
About This Article
Copyright
Data & Comments
Data
 Cite This Article 132 clicks
Cite This Article 132 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.