Enteral immunonutrition in gastric cancer
Gastric cancer (GC), which is a leading contributor to cancer death worldwide, seriously impairs the health basis and decreases the quality of life of patients.[1] Surgery has been the mainstay to curatively treat the GC patients.[2] However, most importantly, various serious postoperative complications (i.e. malnutrition, immune suppression, etc.) will deeply obstacle the prognosis of these given patients.
Sparse evidences published reveal a beneficial of facilitating recovery of patients undergoing the gastrectomy from surgery in enteral immunonutrition (EIN) regime group,[3,4] but there are some studies support standard enteral nutrition (SEN) to be as the nutrition support for patients undergoing surgery for GC.[2] So which nutrition support regimes can be selected to be as the preferred option for GC patients undergoing gastrectomy is still an issue. To address this controversial question, we previously performed a systematic review and meta-analysis of randomized controlled trials (RCTs) investigating comparative effectiveness between EIN and SEN in this specified patients.[5] Our pooled findings on the basis of limited observed events and included studies suggested that clinical outcomes including surgical site infections (SSIs) and other infectious complications (OICs) cannot benefit from EIN regime relative to SEN design.
It is well known that systematic review and meta-analysis of RCTs is a statistical technique to aggregate the data from homogeneous studies and increase the statistical power and precision of the estimated intervention effect through accumulating the observed events and target sample size eventually. It is must note that, however, if the number of accrued patients is smaller than the optimal information size required, meta-analyses include only a limited number of trials and a small number of events may overestimate intervention effect estimates and can cause spurious findings.[6] To overcome this issue, efforts have been done and trial sequential analysis (TSA) is introduced eventually to calculate the required information size (RIS) for a meta-analysis,[7] which is used to determine when a conclusion from a meta-analysis is reliable and conclusive.
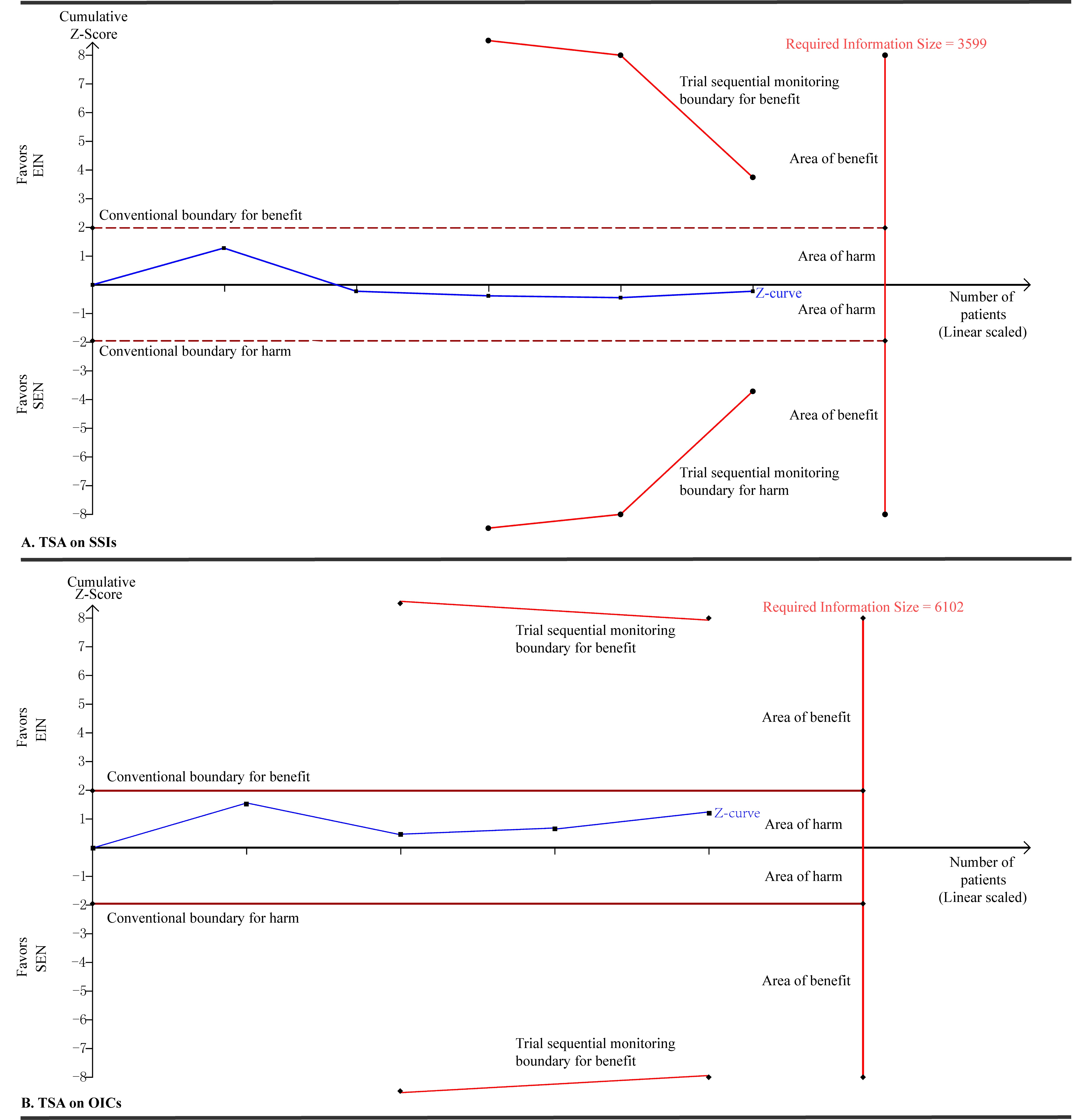
Considered small eligible studies and observed events existed in our study,[5] we adopted consequently TSA technique to determine the robust of our pooled results to avoid spurious conclusion. We calculated the RIS to yield “moderate” meta-analytic evidence based on an α of 0.05 with two sided, β of 0.20 (that is power of 80%), an anticipated relative risk reduction of 20%, and an event proportion of 16.72% and 20.91% in the control arms in terms of SSIs and OICs respectively. TSA on SSIs and OICs in trials with SEN showed that the RISs of 3,599 and 6,102 patients are not reached and cumulative Z-curves are not cross the conventional and TSA monitoring boundaries [Figure 1].
Figure 1. Trial sequential analyses on SSIs and OICs in trials with nutrition support when EIN versus SEN regimes. A diversity adjusted information size of 3,599 (A) and 6,102 (B) patients were calculated using α = 0.05 (two sided), β = 0.20 (power 80%), an anticipated relative risk reduction of 20% and an event proportion of 16.72% and 20.91% in the control arms in terms of SSIs and OICs respectively. TSA illustrated that the cumulative Z-curve did not cross the conventional and TSA monitory boundaries for benefit and that the required information size was not achieved, showing that EIN do cannot improve the clinical status of GC patients undergoing gastrectomy compared to SEN designs. SSIs: surgical site infections; OICs: other infectious complications; EIN: enteral immunonutrition; SEN: standard enteral nutrition; TSA: trial sequential analysis; GC: gastric cancer
The TSA results confirm our pooled findings. In other words, EIN cannot improve the clinical status of patients undergoing surgery for GC. However, the results from our published study showed that EIN is superior to SEN designs in enhancing the host immunity and relieving the inflammatory response,[5] and thus trials with well design are needed to explore whether EIN can improve the degree of infection relative to SEN and the relationship between duration of intervention and incidence of infection.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
Patient consent
No patient involved.
Ethics approval
This article does not contain any studies with human participants or animals.
REFERENCES
1. Anderson WF, Camargo MC, Fraumeni JF Jr., Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA 2010;303:1723-8.
2. Fujitani K, Tsujinaka T, Fujita J, Miyashiro I, Imamura H, Kimura Y, Kobayashi K, Kurokawa Y, Shimokawa T, Furukawa H; Osaka Gastrointestinal Cancer Chemotherapy Study Group. Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer. Br J Surg 2012;99:621-9.
3. Farreras N, Artigas V, Cardona D, Rius X, Trias M, González JA. Effect of early postoperative enteral immunonutrition on wound healing in patients undergoing surgery for gastric cancer. Clin Nutr 2005;24:55-65.
4. Marano L, Porfidia R, Pezzella M, Grassia M, Petrillo M, Esposito G, Braccio B, Gallo P, Boccardi V, Cosenza A, Izzo G, Di Martino N. Clinical and immunological impact of early postoperative enteral immunonutrition after total gastrectomy in gastric cancer patients: a prospective randomized study. Ann Surg Oncol 2013;20:3912-8.
5. Song GM, Tian X, Liang H, Yi LJ, Zhou JG, Zeng Z, Shuai T, Ou YX, Zhang L, Wang Y. Role of enteral immunonutrition in patients undergoing surgery for gastric cancer: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94:e1311.
6. Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, Guyatt G, Devereaux PJ, Thabane L. The number of patients and events required to limit the risk of overestimation of intervention effects in meta-analysis--a simulation study. PLoS One 2011;6:e25491.
7. Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for trial sequential analysis (TSA). Copenhagen, Denmark: Copenhagen Trial Unit, Center for Clinical Intervention Research; 2011. P.1-115. Available from: http://www.ctu.dk/tsa.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Tian X, Song GM, Li Q. Enteral immunonutrition in gastric cancer. J Unexplored Med Data 2016;1:3-5. http://dx.doi.org/10.20517/2572-8180.2016.03
AMA Style
Tian X, Song GM, Li Q. Enteral immunonutrition in gastric cancer. Journal of Unexplored Medical Data. 2016; 1(-1): 3-5. http://dx.doi.org/10.20517/2572-8180.2016.03
Chicago/Turabian Style
Tian, Xu, Guo-Min Song, Qi Li. 2016. "Enteral immunonutrition in gastric cancer" Journal of Unexplored Medical Data. 1, no.-1: 3-5. http://dx.doi.org/10.20517/2572-8180.2016.03
ACS Style
Tian, X.; Song G.M.; Li Q. Enteral immunonutrition in gastric cancer. J. Unexplored. Med. Data. 2016, 1, 3-5. http://dx.doi.org/10.20517/2572-8180.2016.03
About This Article
Copyright
Author Biographies

Data & Comments
Data

 Cite This Article 0 clicks
Cite This Article 0 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.