Salivary carbonic anhydrase, pH and phosphate buffer concentrations as potential biomarkers of caries risk in children
Abstract
Aim: Oral fluid represents a mirror of the body and saliva has the potential to be used in the detection and diagnosis of diseases. The present study aimed to investigate the potential of salivary carbonic anhydrase (CA), salivary pH and phosphate buffer concentration as biomarkers of dental caries in children saliva.
Methods: The study included 120 children of 3-5 years and 13-15 years of age group. Each age group was divided into two subgroups according to risk of dental caries: low and high caries risk groups. Two saliva samples, stimulated and non-stimulated, were collected from each child in all groups and were analyzed for CA, phosphate buffer concentration as well as pH values.
Results: The investigations found significantly higher CA level in saliva samples of low dental caries risk groups children compared to high caries risk groups. Saliva samples from children with low dental caries risk showed significantly higher phosphate buffer concentrations as well as higher pH levels compared to saliva samples from children in high dental caries risk groups.
Conclusion: The results suggest that salivary CA, phosphate buffer concentration and pH values represent potential biomarkers for the estimation of dental caries risk incidence in children, however, further studies with more patients’ samples are recommended to confirm the results.
Keywords
Introduction
Dental caries is a consequence of dental hard tissue dissolution under cariogenic conditions of the dental biofilm. It is considered a complex phenomenon involving internal defense factors, such as saliva, tooth surface morphology, general health, and a number of external factors, e.g. diet, the microbial flora colonizing the teeth, oral hygiene, and fluoride availability.[1] Caries risk assessment is the determination of the likelihood of the incidence of caries during a certain time period or the likelihood that there will be a change in the size or activity of lesions already presents.[2]
An accurate caries risk assessment can identify patients at high caries risk for preventive therapies and improve treatment effectiveness. In particular, the roles of saliva and its biological components have been extensively studied for their possible relevance to dental caries, which is the focus of this study.[3]
Non-invasive salivary analysis has great potential in clinical applications because it contains a wide spectrum of analytics, which can serve as biomarkers for assessment of oral and systemic health. This study intends to clarify the impact of some salivary components on caries risk in children. Salivary factors to be studied are salivary carbonic anhydrase (CA), pH, flow rate and phosphate buffer in both stimulated and non-stimulated saliva.
Salivary buffering, clearance, and flow rate work in concert to influence intraoral pH changes.[4,5] The major regulator of pH is salivary bicarbonate from parotid saliva.[6] The mean or average pH of normal resting saliva is 6.75, which shows that normal resting saliva is slightly acidic.[5,7] Hawkins[8] reported that there was a higher pH in saliva of persons who are immune from caries than in those who are susceptible. This was confirmed by reports from both Vitorino et al.[9] and Zhou et al.[10] showing that a higher pH in saliva of persons who are immune from caries than in those who are susceptible.
Phosphate buffer, together with bicarbonate buffer and proteins, form the basis of the salivary buffer system.[11] Phosphate buffer is a predominant buffer in non-stimulated saliva, and considered essential for the basic, non-stimulated saliva physiology and plays an important role in caries etiopathogenesis.[12]
CA is expressed in most tissues of the human body, participating in pH regulation, carbon dioxide and bicarbonate transport, as well as in the maintenance of water and electrolyte balance.[13-15] Seven isozymes have been identified in mammals, and all are expressed in the alimentary tract.[16] CA VI is a secretory iso-enzyme secreted into the saliva by the serous acinar cells of the human parotid and submandibular glands.[17]
Salivary CA VI is the first salivary protein reported to be associated with the occurrence of caries in individuals.[17] CA VI is believed to provide a greater buffering capacity to saliva by penetrating dental Biofilm and facilitating acid neutralization by salivary bicarbonate.[14] Dušan et al.[18] reported that salivary concentration (activity) of CA VI can definitely be mentioned among the important biomarkers in caries etiopathogenesis.
Methods
Subjects eligibility criteria
Patients included in this study were selected randomly from out-patient clinic of Pediatric Dentistry Department, Faculty of Oral and Dental Medicine, Cairo University and a nursery for staff workers of the University. All subjects in this study were selected according to the following criteria: (1) healthy children (medically free); (2) displays no evidence of significant intra-oral soft tissue disease; (3) co-operative child and parent; and (4) agreement for participation in this research (patient consent form). All procedures and outcomes were explained to parents or to child legal guardians. Patients who accepted to participate in this study had signed an informed consent approved by research ethic committee Faculty of Dentistry Cairo University.
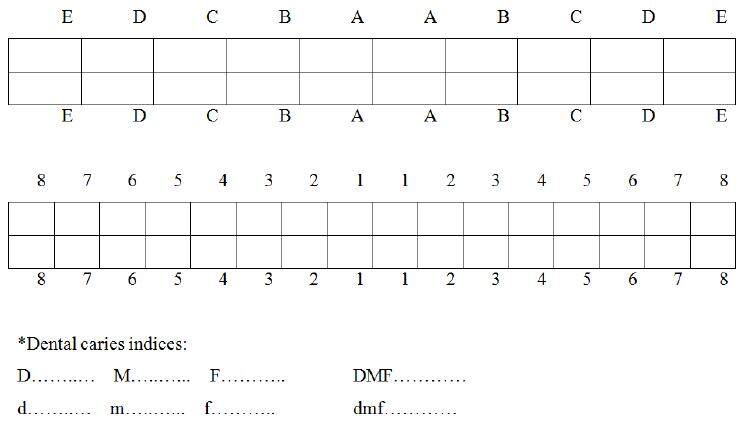
One hundred and twenty children were selected in this and were divided into two groups; group 1 and group 2 comprising of age groups 3-5 years and 13-15 years, respectively. Each of both groups was sub-divided, according to their caries index (dmf\DMF), into low caries risk and high caries risk [Figure 1].
Figure 1. Decayed-Missing-Filled DMF\dmf caries index is one of the most common methods in oral epidemiology for assessing dental caries prevalence among populations. This index is based on in-field clinical examination of individuals by using a probe, mirror and cotton rolls, and simply counts the number of decayed, missing (due to caries only) and restored teeth. dmf: d = decayed; m = missing due to caries; f = filled; DMF: D = decayed; M = missing; F = Filled
Group 1 subdivided into two sub groups based on (dmf) index (d = decayed, m = missing due to caries, f = filled) for deciduous teeth. Subgroup A: high caries risk patients with average dmf (4.5-6.5). Subgroup B: low caries risk patients with average dmf (0-1.1). Group 2 subdivided into two subgroups based on DMF index (D = decayed, M = missing, F = Filled). Subgroup A: high caries index with average DMF (4.5-6.5). Subgroup B: low caries index with average DMF (0-1.1).
All children participated in this study were examined for the following.
Clinical examination
Dental examination: dental caries experience in permanent dentition and primary dentition using DMF caries index for permanent teeth, and dmf caries index for primary teeth using the World Health Organization criteria.[19] All children asked to fast for at least 2 h prior to saliva collection.
Sample collection [Figure 2]: (1) non-stimulated salivary flow rate (mL/min) (spitting method); and (2) stimulated salivary flow rate (mL/min) (masticatory stimulation method using paraffin wax).
Laboratory investigation
Non-stimulated and stimulated saliva was collected from each group by spitting method and paraffin wax respectively. The flow rate was estimated by asking patient to spit in the graduated tube for 5 min. Samples were used for measuring the following salivary factors: (1) salivary pH using pH 212 microprocessor pH Meter, HANNA instruments, USA; (2) salivary phosphate buffer concentrations using Colorimetric ab65622 (abcam); and (3) salivary CA by Ericsson method. Each salivary factor was correlated individually with the dental caries index of both primary and permanent dentition to establish the effect of these factors on dental caries experience.
Statistical analysis
Statistical analysis was performed with IBM®SPSS® Statistics Version 21 for Windows® SPSS, IBM. Difference between tested groups were analyzed using one-way ANOVA followed Tukey’s post-hoc test for pair-wise comparison between the means when ANOVA test is significant.
Results and discussion
In this prospective study, 120 children belonging to age 3-5 and 13-15 years were selected by using stratified sampling procedure, (60 children for each age group), this classification was in agreement with Surdilović et al.[20] This age group was chosen to evaluate the role of various salivary factors in relation to caries incidence in both primary and early permanent dentition. Whole non-stimulated and stimulated saliva were tested in this study.[20] Parotid, submandibular or sublingual saliva were not tested as it is difficult to obtain saliva directly from salivary gland ducts in children. Also whole saliva is considered a reflection of all salivary secretions rather than saliva of a specific gland as reported by Malamud et al.[21] The saliva was collected at morning to prevent circadian variation and the participants fasted for at least 2 h before saliva collection to avoid influence of immediate food consumption and foul contamination.[11,12,20]
We found significant differences in CA activities in relation to the level of risk of caries. Our results showed that patients in the low caries risk group had significantly higher CA activity in their saliva comparing to children with high risk of caries. In the young age group (3-5 years), the high-risk group showed the lowest significant (P ≤ 0.001) CA mean values (3.87 ± 1.47) compared to the low caries risk group (6.75 ± 0.44) for non-stimulated samples. The same trend was observed in the stimulated samples where high-risk group also showed the lowest significant (P ≤ 0.001). CA mean values (4.66 ± 1.56) compared to the low caries risk group (8.37 ± 0.8). Following the same pattern in the young age groups non-stimulated samples from high-risk group age (13-15 years) showed the lowest significant (P ≤ 0.001) CA mean values (3.53 ± 1.28) compared to the low caries risk group (6.97 ± 1.16). Stimulated samples from the same age group also showed the same pattern where the high-risk group showed the lowest significant (P ≤ 0.001) CA mean values (4.76 ± 0.99) compared to the low caries risk group (8.24 ± 1.3). Mean and standard deviation (SD) of CA for different groups tested are presented [Table 1 and Figure 3].
Salivary carbonic anhydrase concentration measured in stimulated and non-stimulated salivary samples of patients with different caries risk groups (mean ± SD)
| Groups | 13-15 years | 3-5 years | P-value | |||
|---|---|---|---|---|---|---|
| Low caries risk | High caries risk | Low caries risk | High caries risk | |||
| Salivary carbonic anhydrase | Non-stimulated | 6.97a ± 1.16 | 3.53b ± 1.28 | 6.75a ± 0.44 | 3.87b ± 1.47 | ≤ 0.001 |
| Stimulated | 8.24a ± 1.30 | 4.76b ± 0.99 | 8.37a ± 0.80 | 4.66b ± 1.56 | ≤ 0.001 | |
| P-value | 0.009 | ≤ 0.001 | 0.007 | 0.166 | ||
Figure 3. Histogram showing the mean of carbonic anhydrase concentration measured in stimulated and non-stimulated salivary samples of patients with different caries risk groups
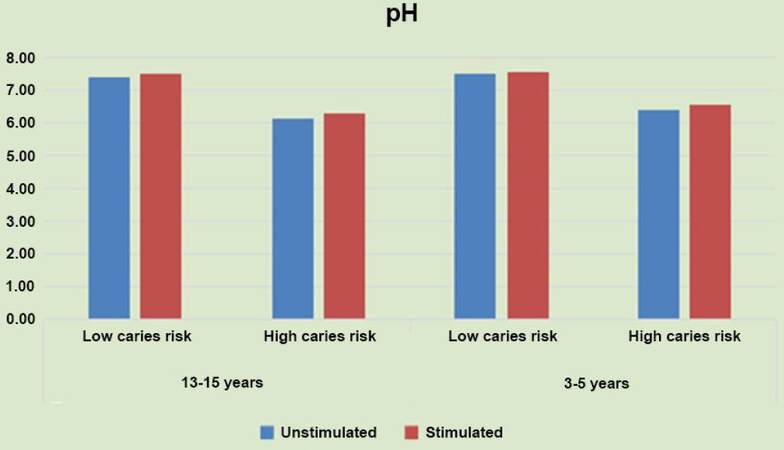
Our results also showed that pH level exhibited a significant negative correlation with the level of risk of caries. Measurement of pH in young age groups (3-5 years) showed that the high-risk group exhibited the lowest significant (P ≤ 0.001) mean pH values (6.4 ± 0.22) compared to the low caries risk group (7.5 ± 0.19) as measured in the non-stimulated samples. The same trend was observed in the stimulated samples where high-risk group also showed the lowest significant pH mean values (6.18 ± 0.12) compared to the low caries risk group (7.4 ± 0.2). The high-risk group showed the highest insignificant mean pH values (6.59 ± 0.25) compared to the low caries risk group (7.6 ± 0.28). The same pattern was observed in the older age group (13-15 years) where the high-risk group showed the lowest significant (P ≤ 0.001) pH mean values (6.18 ± 0.12) compared to the low caries risk group (7.4 ± 0.2). In the stimulated samples, the high-risk group also showed the lowest significant (P ≤ 0.001) pH mean values (6.3 ± 0.18) compared to the low caries risk group (7.51 ± 0.23). Mean and SD for pH for different groups tested are presented [Table 2 and Figure 4].
pH values measured in stimulated and non-stimulated salivary samples of patients with different caries risk groups (mean ± SD)
| Groups | 13-15 years | 3-5 years | P-value | |||
|---|---|---|---|---|---|---|
| Low caries risk | High caries risk | Low caries risk | High caries risk | |||
| pH | Non-stimulated | 7.40a ± 0.20 | 6.18c ± 0.12 | 7.50a ± 0.19 | 6.40b ± 0.22 | ≤ 0.001 |
| Stimulated | 7.51a ± 0.23 | 6.30c ± 0.18 | 7.60a ± 0.28 | 6.59b ± 0.25 | ≤ 0.001 | |
| P-value | 0.184 | 0.259 | 0.042 | 0.039 | ||
Figure 4. Histogram of the pH mean values measured in stimulated and non-stimulated salivary samples of patients with different caries risk groups
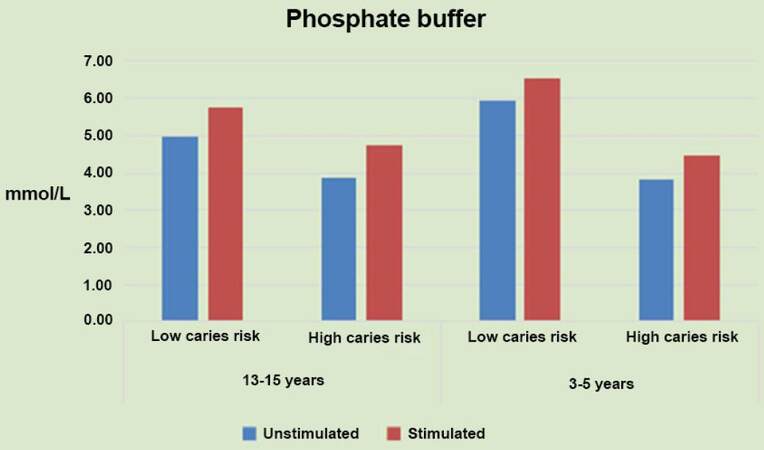
Phosphate buffer level has followed the same pattern of CA and pH in beaing in negative correlation with the level of risk of caries. The low risk group patients (3-5 years) age group showed the highest significant (P ≤ 0.001) phosphate buffer mean values (5.94 ± 1.67) compared to the high caries risk group (3.84 ± 0.42) as measured in the non-stimulated samples. The same pattern was verified in the stimulated samples where the low risk group showed also the highest significant mean phosphate buffer values (6.56 ± 1.47) compared to the high caries risk group (4.47 ± 0.61). The same pattern was observed in the older (13-15 years) age group, where the low risk group showed the highest significant (P = 0.005) phosphate buffer mean values (4.99 ± 1.34) compared to the high caries risk group (3.86 ± 0.49) when measured in non-stimulated samples. In the stimulated samples the low risk group showed also the highest significant (P = 0.016) phosphate buffer mean values (5.75 ± 1.35) compared to the high caries risk group (4.76 ± 0.66). SD for phosphate buffer for different groups tested is presented [Table 3 and Figure 5].
Phosphate buffer concentration measured in stimulated and non-stimulated salivary samples of patients with different caries risk groups (mean ± SD)
| Groups | 13-15 years | 3-5 years | P-value | |||
|---|---|---|---|---|---|---|
| Low caries risk | High caries risk | Low caries risk | High caries risk | |||
| Phosphate buffer | Non-stimulated | 4.99c ± 1.34 | 3.86b ± 0.49 | 5.94c ± 1.76 | 3.84a ± 0.42 | ≤ 0.001 |
| Stimulated | 5.75c ± 1.35 | 4.76b ± 0.66 | 6.56c ± 1.47 | 4.47a ± 0.51 | ≤ 0.001 | |
| P-value | ≤ 0.003 | 0.001 | 0.291 | 0.131 | ||
Figure 5. Histogram of the mean of phosphate buffer levels measured in stimulated and non-stimulated salivary samples of patients with different caries risk groups
Our results are in agreement with the results of several studies[19,21,22] that reported that low salivary concentration of CA has been related to increased caries prevalence. It has been reported that CA may protect the enamel surface by catalyzing the most important buffer system in the oral cavity, thus accelerating the neutralization of acid from the local environment of the tooth surface, moreover, it has been demonstrated that salivary CA may accumulate in the enamel pellicle and function as a local pH regulator on the enamel surface and thus would help to prevent dental caries.[19,21-23]
On the other hand, some of our results were not in agreement with other authors.[23-25] This conflict may be due to the difference in method of analysis as our study were able to determine the concentration of salivary CA VI while Frasseto et al.[25] used the zymography method to quantitatively determine the activity of salivary CA VI.[24] Their results indicate significantly higher activity of CA in stimulated than non-stimulated saliva in both examined groups, while Surdilović et al.[20] reported a positive correlation between CA VI concentration and saliva secretion.[26] It should be realized that several factor affect the saliva composition that could changes according to the flow rate, nature and duration of stimulation, and the time of day at which samples are collected. In many studies, these variables have not been adequately taken into account and this may explain the variability in the results of different studies. Consequently, the need for standardization of normal values becomes increasingly apparent when saliva is used for diagnosis. Further studies with more patients’ samples are recommended to determine concentration and activity of CA isozyme in the saliva of preschool children with caries and to investigate the relationship between caries and salivary CA activity, salivary flow rate, phosphate buffer and pH.
Acknowledgments
The authors thank the patients whose participation made this study possible. They would also like to thank Dr. M. Saad for his scientific guidance and the help in preparing the manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Patient consent
Patient consent was obtained from the patients.
Ethics approval
Ethics approval was obtained prior to the commencement of the study.
REFERENCES
1. Llena C, Forner L. Dietary habits in a child population in relation to caries experience. Caries Res 2008;42:387-93.
2. Council on Clinical Affairs. Guideline on caries-risk assessment and management for infants, children, and adolescents. Am Acad Pediatr Dent 2014;37:132-9.
3. Braga MM, Oliveira LB, Bonini GA, Bönecker M, Mendes FM. Feasibility of the international caries detection and assessment system (ICDAS-II) in epidemiological surveys and comparability with standard World Health Organization criteria. Caries Res 2009;43:245-9.
4. Humphrey SP, Williamson RT. A review of saliva: normal composition, flow and function. J Prosthet Dent 2001;85:162-9.
6. Moritsuka M, Kitasako Y, Burrow MF, Ikeda M, Tagami J. The pH change after HCI titration into resting and stimulated saliva for a buffering capacity test. Aust Dent J 2006;51:170-4.
8. Hawkins HF. A rational technique for the control of caries and systemic pyorrhea. J Dent Res 1931;11:201-34.
9. Vitorino R, Calheiros-Lobo MJ, Duarte JA, Domingues P, Amado F. Salivary clinical data and dental caries susceptibility: is there a relationship? Bull Group Int Rech Sci Stomatol Odontol 2006;47:27-33.
10. Zhou Q, Bai J, Qin M. Relationship between cariogenic microbe, salivary buffer capacity and early childhood caries. Zhonghua Kou Qiang Yi Xue Za Zhi 2007;42:581-4.
11. Preethi BP, Reshma D, Anand P. Evaluation of flow rate, pH, buffering capacity, calcium, total proteins and total antioxidant capacity levels of saliva in caries free and caries active children: an in vivo study. Indian J Clin Biochem 2010;25:425-8.
12. Poureslami HR, Torkzadeh M, Sefadini MR. Study of changes in phosphate, calcium and fluoride ions in plaque and saliva after administration of fluoride mouth rinse. J Indian Soc Pedod Prev Dent 2007;25:122-5.
13. Supuran CT, Scozzafava A. Applications of carbonic anhydrase inhibitors and activators in therapy. Expert Opin Ther Pat 2002;12:217-42.
14. Kimoto M, Kishino M, Yura Y, Ogawa Y. A role of salivary carbonic anhydrase VI in dental plaque. Arch Oral Biol 2006;51:117-22.
15. Arabacı T, Çiçek Y, Beydemir S, Çanakçı CF, Çanakçı V. Are increased salivary carbonic anhydrase VI levels related to the amount of supragingival dental calculus formation and clinical periodontal scores? J Dent Sci 2015;10:123-7.
16. Parkkila S, Parkkila AK. Carbonic anhydrase in the alimentary tract. Roles of the different isozymes and salivary factors in the maintenance of optimal conditions in the gastrointestinal canal. Scand J Gastroenterol 1996;31:305-17.
17. Kivelä J, Parkkila S, Parkkila A, Leinonen J, Rajaniemi H. Salivary carbonic anhydrase isoenzyme VI. J Physiol 1999;520:315-20.
18. Dušan S, Ivana S, Mirjana A, Marija I. The role of carbon anhydrase in the occurrence of caries. Acta Stomatologica Naissi 2008;24:789-92.
19. Szabَ I. Carbonic anhydrase activity in the saliva of children and its relation to caries activity. Caries Res 1974;8:187-91.
20. Surdilović D, Stojanović I, Apostolović M. Caries risk estimation in children regarding values of saliva buffer system components and carbon hydrase activity. Vojnosanit Pregl 2008;65:676-80.
21. Malamud D, Abrams WR, Bau H, Wang J, Chen Z, Corstjens P, Niedbala S. Oral-based techniques for the diagnosis of infectious diseases. J Calif Dent Assoc 2006;34:297-301.
22. Englander HR, Mau LM, Hoerman KC, Chauncey HH. Dental caries activity, and the pH, titrable alkalinity, and rate of flow of human parotid saliva. J Dent Res 1958;37:906-11.
23. Leinonen J, Kivelä J, Parkkila S, Parkkila AK, Rajaniemi H. Salivary carbonic anhydrase isoenzyme VI is located in the human enamel pellicle. Caries Res 1999;33:185-90.
24. Oztürk LK, Furuncuoğlu H, Atala MH, Uluköylü O, Akyüz S, Yarat A. Association between dental-oral health in young adults and salivary glutathione, lipid peroxidation and sialic acid levels and carbonic anhydrase activity. Braz J Med Biol Res 2008;41:956-9.
25. Frasseto F, Parisotto TM, Peres RC, Marques MR, Line SR, Nobre Dos Santos M. Relationship among salivary carbonic anhydrase VI activity and flow rate, biofilm pH and caries in primary dentition. Caries Res 2012;46:194-200.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Makawi Y, El-Masry E, El-Din HM. Salivary carbonic anhydrase, pH and phosphate buffer concentrations as potential biomarkers of caries risk in children. J Unexplored Med Data 2017;2:9-15. http://dx.doi.org/10.20517/2572-8180.2016.07
AMA Style
Makawi Y, El-Masry E, El-Din HM. Salivary carbonic anhydrase, pH and phosphate buffer concentrations as potential biomarkers of caries risk in children. Journal of Unexplored Medical Data. 2017; 2: 9-15. http://dx.doi.org/10.20517/2572-8180.2016.07
Chicago/Turabian Style
Makawi, Yasmine, Eman El-Masry, Hala Mohye El-Din. 2017. "Salivary carbonic anhydrase, pH and phosphate buffer concentrations as potential biomarkers of caries risk in children" Journal of Unexplored Medical Data. 2: 9-15. http://dx.doi.org/10.20517/2572-8180.2016.07
ACS Style
Makawi, Y.; El-Masry E.; El-Din HM. Salivary carbonic anhydrase, pH and phosphate buffer concentrations as potential biomarkers of caries risk in children. J. Unexplored. Med. Data. 2017, 2, 9-15. http://dx.doi.org/10.20517/2572-8180.2016.07
About This Article
Copyright
Author Biographies

Data & Comments
Data
 Cite This Article 12 clicks
Cite This Article 12 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.