Safety and efficacy of biweekly cetuximab based chemotherapy for patients with metastatic colorectal cancer
Abstract
Aim: Cetuximab was administered weekly in registration clinical trials. Biweekly administration is more convenient when combining cetuximab with biweekly chemotherapy in patients with metastatic colorectal cancer (mCRC). The aim of this study is to evaluate safety and efficacy of biweekly cetuximab at a dose of 500 mg/m2 with chemotherapy in routine clinical practice.
Methods: Clinical data of 19 consecutive patients with K-RAS wild type mCRC who received biweekly cetuximab with biweekly fluropyrimidine based chemotherapy were reviewed. Toxicity assessment was limited to the first 6 cycles of treatment. Best tumor response was assessed by an independent radiologist.
Results: Median age was 59 (24-74) years. Cetuximab was administered in first, second and third line settings in 7, 9 and 3 patients respectively. Grade I/II cetuximab specific adverse events (AEs) were skin rash (47.3%), diarrhea (21%), infusion reactions (10.5%), Hypomagnesaemia (10.5%) and nail disorders (5%). Grade III AEs were skin rash (10.5%) and diarrhea (5.3%). There was no grade IV AEs. There were no complete responders. Partial response was achieved in 8 (42.1%) and stable disease in 6 (31.5%) patients.
Conclusion: This small but real life experience shows that biweekly cetuximab with chemotherapy is safe and effective. The frequency of AEs compares favorably to weekly administration reported in the literature. These finding add to the relatively limited available data on biweekly administration to support its adoption in routine clinical practice.
Keywords
Introduction
Colorectal cancer (CRC) is the third most common type of malignancy diagnosed in the US. It is one of the leading causes of cancer mortality in men and women.[1] Some countries are experiencing slight declines in the incidence possibly due to screening.[2] However, others are reporting a significant rise in reported cases. The 2011 National Saudi Cancer Registry reported 58% rise in the number of diagnosed cases when compared to the 2004 registry report.[3] The United Kingdom reports a 5% rise in the years between 2000/2002 and 2009/2011.[4]
Approximately 25% of patients with CRC present with metastatic disease and 40-50% develop metastases after initial curative intent treatment. The outcome of patients with metastatic CRC (mCRC) is poor. However, the recent 2 decades have seen successful development of newer and more effective chemotherapy and targeted agents leading to measurable improvement in outcome.[5]
Cetuximab, an IgG1 monoclonal antibody against the epidermal growth factor receptor (EGFR) improves the outcome of patients with rat sarcoma (RAS) wild type tumors when combined with chemotherapy.[6] The approved schedule of cetuximab consists of a first loading dose of 400 mg/m2 followed by 250 mg/m2 per week. Meanwhile, most chemotherapy regimens for the treatment of mCRC are administered biweekly. This creates frequent visits to the hospital and extra costs. It would be more convenient both for the patient and for the treating institution if cetuximab could be administered every 2 weeks. Pharmacokinetic, Pharmacogenomic and pharmacoproteomic studies show equivalence of weekly and biweekly (500 mg/m2) administration.[7]
Encouraged by these findings and by those of few clinical phase I/II studies, we adopted a biweekly regimen for administration of cetuximab in routine clinical practice. Here we report our initial experience with this schedule coupled with planned and structured evaluation to guide our future practice.
Methods
The analysis included the first 19 consecutive patients after the adoption of biweekly administration of cetuximab. All patients had K-RAS wild type mCRC and received cetuximab based therapy in combination with chemotherapy in first, second or third line settings. There were no predefined inclusion and exclusion criteria. All patients were selected and managed according to routine standard care at the institution.
Treatment and toxicity details were collected prospectively during each treatment cycle on a data collection form (DCF) for each patient. Treatment details included chemotherapy regimen and dose adjustment. Relevant toxicity thought to be caused by cetuximab was captured. Specific cytotoxic chemotherapy adverse events were not captured (i.e. hand foot syndrome). Toxicity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Standard measures were employed to manage treatment related adverse events. Structured toxicity assessment and documentationon DCF was limited to the first 6 cycles of treatment and is presented in this report.
Cetuximab was administered at a dose of 500 mg/m2 every 2 weeks with standard premedication to reduce risk of infusion-related reactions. Reduction of cetuximab dose to 400 mg/m2 and 250 mg/m2 was employed for patients experiencing grade III or more toxicity.
Concomitant chemotherapy was also administered every 2 weeks. Capecitabine with oxaliplatin (XELOX) consisted of capecitabine 1,250 mg/m2 twice a day for 9 days and oxaliplatin 85 mg/m2 on day 1. Capecitabine with irinotecan (XELIRI) consisted of capecitabine 1,250 mg/m2 twice a day for 9 days and irinotecan 180 mg/m2 on day 1. Patients continued treatment until evidence of progressive disease or development of intolerable side effects.
There was no predefined response assessment protocol. However, and as per routine practice, all patients underwent computed tomography scan radiological assessment every 6-8 weeks. Response rate was assessed according to Response Evaluation Criteria in Solid Tumors v.1.0 by an independent expert radiologist. The best radiological response during treatment was documented as the response to treatment. Progression free survival (PFS) was calculated from start of cetuximab based therapy (CBT) until progression or date of last follow up. Overall survival (OS) was calculated from start of CBT until death or date of last follow up. Statistical Package for the Social Sciences 11.5 software was used for data analysis. Time related progression and survival events were analyzed using Kaplan-Meier analysis.
Results
Nineteen patients (10 males and 9 females) were treated and included in this analysis. Median age was 59 (24-74) years. Treatment was administered as first, second and third line settings in 7 (36.8%), 9 (47.4%) and 3 (15.8%) patients respectively. Fourteen patients received concomitant XELIRI, 6 (42.9%) of whom achieved partial response (PR). Five patients received concomitant XELOX, 2 (40%) of whom achieved PR [Table 1]. There were no complete responses.
Radiological objective response to CBT
| All patients n = 19 | XELIRI &cetuximab n = 14 | XELOX &cetuximab n = 5 | First line n = 7 | Second line n = 9 | Third line N = 3 | |
|---|---|---|---|---|---|---|
| PR | 8 (42.1%) | 6 (42.9%) | 2 (40%) | 4 (57%) | 4 (44%) | 0 (0%) |
| SD | 6 (31.5%) | 4 (28.5%) | 2 (40%) | 2 (28.6%) | 3 (33.3%) | 1 (33.3%) |
| PD | 5 (26.3%) | 4 (28.5%) | 1 (20%) | 1 (14.3%) | 2 (22%) | 2 (66.7%) |
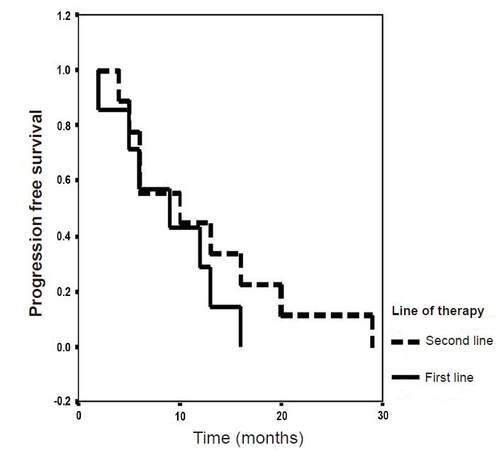
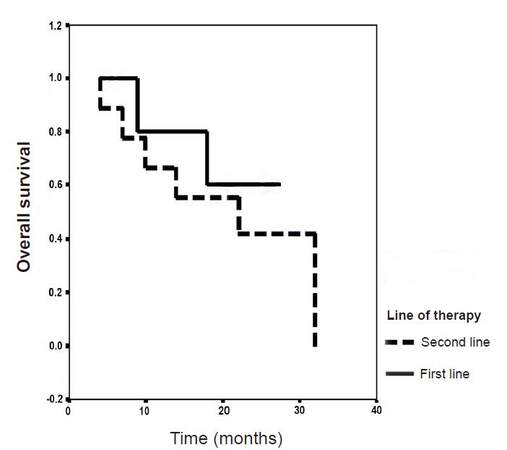
Median PFS was 9 and 10 months in patients who received cetuximab in first and second line settings respectively [Figure 1]. Three patients received treatment in third line setting and progressed after 4, 7 and 24 months. Median OS was not reached and was 22 months in patients who received cetuximab in first and second line settings respectively [Figure 2]. Three patients received treatment in third line setting and died after 8, 12 and 39 months.
Skin rash and diarrhea were the most common adverse events (AEs) expected to be related to cetuximab treatment and were reported in 11 (57.9%) and 5 (26.3%) patients respectively. Grade III AEs were observed in only 3 (15.8%) patients including skin rash (2 patients) and diarrhea (1 patient). There was no grade IV AEs. Table 2 depicts the details of cetuximab related AEs. Three (15.8%) patients had dose reduction of cetuximab to 400 mg/m2. Two of them required further dose reduction to 250 mg/m2.
Adverse events related to cetuximab
| Toxicity | Grade I/II (%) | Grade III (%) |
|---|---|---|
| Skin rash | 9 (47.3%) | 2 (10.5%) |
| Diarrhea | 4 (21%) | 1 (5.3%) |
| Infusion reaction | 2 (10.5%) | 0 |
| Fatigue | 2 (10.5%) | 0 |
| Nail changes | 1 (5.3%) | 0 |
| Hypomagnesaemia | 2 (10.5%) | 0 |
| Nausea | 2 (10.5%) | 0 |
| Vomiting | 1 (5.3%) | 0 |
In total, 104 cycles of CBT were administered. Grade I/II neutropenia was reported in 28 (27%) and grade III/IV in 6 (5.8%) cycles. There was no any grade thrombocytopenia reported.
Discussion
Randomized clinical trials confirmed the efficacy of first line cetuximab in combination with chemotherapy in patients with RAS wild type mCRC.[6,8] In addition, its benefit is confirmed as a single agent in patients who progressed on fluropyrimidine, oxaliplatin and irinotecan.[9,10]
Patients in these large (including registration) trials received cetuximab every week leading to the adoption of this schedule in routine practice. Synchronizing the administration of chemotherapy and cetuximab will reduce patients’ visits to hospital and allows best use of resources. The pharmacokinetic and small limited clinical data suggest the feasibility of biweekly dosing.
Our findings are based on unselected patients managed in routine day to day practice setting. The results show that the clinical efficacy of biweekly cetuximab is in line with what is expected from weekly scheduling. In our patients, response rate was 57%, median PFS was 9 months and the median OS was not reached (> 22 months) in patients who received cetuximab in the first line setting [Figures 1 and 2]. These findings are comparable to those of the 2 land marks randomized, OPUS and CRYSTAL first line trials in wild-type K-RAS population. In the combined analysis of these 2 trials, response rate was 57.3%, PFS was 9.9 months and OS was 23.5 months.[6]
In the second line setting 44% of our patients achieved objective response with median PFS and OS of 10 and 22 months respectively [Figures 1 and 2]. This compare favorably to those of weekly cetuximab reported in the literature. The phase II second line FLIER trial reported tumor response in 31.7% of patients with median PFS and OS of 7.4 and 18.2 months respectively with weekly second line cetuximab and 5-fluorouracil-irinotecan (FOLFIRI).[11]
Any grade skin rash and diarrhea occurred in 57.9% and 26.3% of our patients respectively. Grade 3 AEs were observed in only 3 (15.8%) patients including skin rash (2 patients) and diarrhea (1 patient) and there was no grade IV AEs. This toxicity profile of biweekly cetuximabis comparable to that reported in phase III trials of weekly schedule. Any grade rash and diarrhea was reported in 53% (GIII/IV: 16%) and 84% (GIII/IV: 26%) when cetuximab was combined with FOLFX in the OPUS trial.[12] The CRYSTAL trial investigated cetuximab in combination with FOLFIRI. Grade I/II AEs were not reported in the CRYSTAL publication. However, GIII/IV rash and diarrhea was reported in 16.2% and 15.7% of patients.[13]
In a retrospective study, Chen et al.[14] reviewed 24 patients who received biweekly cetuximab plus FOLFIRI. Response rate was 50.0% and 33.3% and median PFS was 8.8 and 4.6 months in first and second line settings respectively. Rash was observed in 69.2% of evaluable patients (G III: 7.7%). In another retrospective study, Mrabti et al.[15] reviewed 50 heavily pre-treated patients. Cetuximab was administered weekly in 32 and biweekly in 18 patients. Objective response was 28.1% and 11.1% while disease control (partial response and stable disease) was 56.2% and 78.1% for weekly and biweekly schedules respectively. Additionally there was no statistically difference in PFS and OS between both groups. Rash was reported in 78.1% (GIII: 3.1% and GIV: 0%) and 61% (GIII: 5.5% and GIV: 0%) while gastrointestinal AEs including diarrhea in 40.6% (GIII/IV: 3.1%) and 55.5% (GIII/IV: 5.5%) with weekly and biweekly schedules respectively.
Few prospective single arm trials investigated biweekly cetuximab. The largest are the NORDIC-7.5 (plus irinotecan based chemotherapy in 152 patients) and the OPTIMIX-ACROSS (plus oxaliplatin based chemotherapy in 99 patients) trials. Both trials concluded that the efficacy and toxicity of the convenient biweekly cetuximab is consistent with that of weekly schedule.[16,17]
To our knowledge, the CECOG trial is the only relatively large randomized that prospectively randomized patients to both schedules. Patients received FOLFOX plus either weekly (arm 1: n = 75) or biweekly (arm 2: n = 77) cetuximab (500 mg/m2) until disease progression or unacceptable toxicity. In arms 1 and 2, objective response (53% vs. 62%, Odds Ratio: 1.40, 95% Confidence Interval (CI): 0.74-2.66), PFS (median 9.5 vs. 9.2 months, (Heart Rate) HR: 0.92, 95% CI: 0.63-1.34), OS (median 25.8 vs. 23.0 months, HR: 0.86, 95% CI: 0.56-1.30) and disease control (87% vs. 87%) respectively were comparable. Rash was reported in 64% (GIII/IV: 19%) and 68% (GIII/IV: 27%) while diarrhea in 35% (GIII/IV: 8%) and 30% (GIII/IV: 10%) in arms 1 and 2 respectively. The authors concluded that the activity and safety of FOLFOX plus either cetuximab administered weekly or biweekly were similar.[18]
In the Medical Research Council COIN trial, 1,630 patients were randomly assigned to treatment groups: 815 to oxaliplatin and 5 FU chemotherapy (FOLFOX or XELOX; arm A) and 815 to the same combinations plus cetuximab (arm B). Tumour samples from 1,316 (81%) patients were used for somatic molecular analyses; 565 (43%) had K-RAS mutations. In patients with K-RAS wild-type tumours (arm A, n = 367; arm B, n = 362), OS did not differ between treatment groups (median survival 17.9 months in the control group vs. 17 months in the cetuximab group; HR 1.04, 95% CI 0.87-1.23, P = 0.67). Similarly, there was no effect on PFS (8.6 months in the control group vs. 8.6 months in the cetuximab group; HR 0.96, 0.82-1.12, P = 0.60). Overall response rate increased from 57% (n = 209) with chemotherapy alone to 64% (n = 232) with addition of cetuximab (P = 0.049).[19] One possible interpretation of these results was that either capectabine (as opposed to 5F) and/or oxaliplatin (as opposed to irinotecan) are not the most effective drugs to combine with cetuximab. There is no clear biochemical evidence that substantiates this interpretation. Authors of the COIN trial report explained that the addition of cetuximab resulted in reduced dose intensity in K-RAS wild-type patients over the first 24 weeks (for 5 FU-based therapy: median 78% in the control group vs. 73% in the cetuximab group, P = 0.031; for capecitabine-based therapy: 85% vs. 79%, P = 0.0021).
This is interpretation has become less popular when the results of the CALGB/SWOG 80405 trial were presented showing no significant difference in OS between cetuximab and bevacizumab regardless of the chemotherapy backbone (oxaliplatin or irinotecan based).[20] One plausible explanation to the COIN results is the imbalance in the patients with other K-ras and N-ras mutant tumors among both treatment arms.
Mutations at codons 12 and 13 of exon 2 of K-RAS are predictive of lack of response to anti-EGFR therapy in advanced stage disease. Recently, K-RAS mutations at codons 59 and 61 of exon 3 and codons 117 and 146 of exon 4 have been identified in 6% and 9% of tumors from patients with advanced CRC respectively. In addition, mutations at exon 2, 3 and 4 of N-RAS have also been identified in 7, 5 and 1% of these patients respectively. These additional K-RAS and N-RAS mutations have also been found to confer resistance to anti-EGFR therapy.[21]
Regardless of the above speculations, intravenous 5 FU by oral capecitabine remains a popular and a practical approach.
The findings of our study add to and support existing pharmacokinetic, pharmacodynamics and clinical data to substantiate the tolerability and efficacy of 500 mg/m2 biweekly cetuximab for patients with mCRC in first and subsequent lines. The currently available evidence supports the adoption of this convenient schedule in routine clinical practice.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
Patient consent
Consent not required as treatment was not investigational.
Ethics approval
The local institutional review board approved the study.
REFERENCES
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108.
2. Siegel RL, Ward EM, Jemal A. Trends in colorectal cancer incidence rates in the United States by tumor location and stage, 1992-2008. Cancer Epidemiol Biomarkers Prev 2012;21:411-6.
3. Cancer Registry reports [Internet]. [cited 2016 Jan 29]. Available from: http://www.chs.gov.sa/En/HealthRecords/CancerRegistry/Pages/CancerRegistryRecords.aspx.
4. Cancer incidence for common cancers | Cancer Research UK [Internet]. [cited 2016 Jan 25]. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/common-cancers-compared#heading-Three.
5. Mitry E, Rollot F, Jooste V, Guiu B, Lepage C, Ghiringhelli F, Faivre J, Bouvier AM. Improvement in survival of metastatic colorectal cancer: are the benefits of clinical trials reproduced in population-based studies? Eur J Cancer 2013;49:2919-25.
6. Bokemeyer C, Van Cutsem E, Rougier P, Ciardiello F, Heeger S, Schlichting M, Celik I, Köhne CH. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer 2012;48:1466-75.
7. Tabernero J, Ciardiello F, Rivera F, Rodriguez-Braun E, Ramos FJ, Martinelli E, Vega-Villegas ME, Roselló S, Liebscher S, Kisker O, Macarulla T, Baselga J, Cervantes A. Cetuximab administered once every second week to patients with metastatic colorectal cancer: a two-part pharmacokinetic/pharmacodynamic phase I dose-escalation study. Ann Oncol 2010;21:1537-45.
8. Van Cutsem E, Köhne C-H, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408-17.
9. Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, Tebbutt NC, van Hazel G, Wierzbicki R, Langer C, Moore MJ. Cetuximab for the Treatment of Colorectal Cancer. N Engl J Med 2007;357:2040-8.
10. Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757-65.
11. Iwamoto S, Hazama S, Kato T, Miyake Y, Fukunaga M, Matsuda C, Bando H, Sakamoto J, Oba K, Mishima H. Multicenter phase II study of second-line cetuximab plus folinic acid/5-fluorouracil/irinotecan (FOLFIRI) in KRAS wild-type metastatic colorectal cancer: the FLIER study. Anticancer Res 2014;34:1967-73.
12. Tabernero J, Van Cutsem E, Díaz-Rubio E, Cervantes A, Humblet Y, André T, Van Laethem JL, Soulié P, Casado E, Verslype C, Valera JS, Tortora G, Ciardiello F, Kisker O, de Gramont A. Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2007;25:5225-32.
13. Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337-45.
14. Chen Y, Cao D, Bi F, Li Q, Qiu M. Biweekly cetuximab plus FOLFIRI/irinotecan as first/second-line chemotherapy for patients with KRAS wild-type metastatic colorectal cancer: a retrospective analysis in Southwest Chinese population. Med Oncol 2014;31:935.
15. Mrabti H, De la Fouchardiere C, Desseigne F, Dussart S, Negrier S, Errihani H. Irinotecan associated with cetuximab given every 2 weeks versus cetuximab weekly in metastatic colorectal cancer. J Cancer Res Ther 2009;5:272-6.
16. Pfeiffer P, Sorbye H, Qvortrup C, Karlberg M, Kersten C, Vistisen K, Lindh B, Bjerregaard JK, Glimelius B. Maintenance therapy with cetuximab every second week in the first-line treatment of metastatic colorectal cancer: the NORDIC-7.5 study by the Nordic Colorectal Cancer Biomodulation Group. Clin Colorectal Cancer 2015;14:170-6.
17. Fernandez-Plana J, Pericay C, Quintero G, Alonso V, Salud A, Mendez M, Salgado M, Saigi E, Cirera L; ACROSS Study Group. Biweekly cetuximab in combination with FOLFOX-4 in the first-line treatment of wild-type KRAS metastatic colorectal cancer: final results of a phase II, open-label, clinical trial (OPTIMIX-ACROSS Study). BMC Cancer 2014;14:865.
18. Brodowicz T, Ciuleanu TE, Radosavljevic D, Shacham-Shmueli E, Vrbanec D, Plate S, Mrsic-Krmpotic Z, Dank M, Purkalne G, Messinger D, Zielinski CC. FOLFOX4 plus cetuximab administered weekly or every second week in the first-line treatment of patients with KRAS wild-type metastatic colorectal cancer: a randomized phase II CECOG study. Ann Oncol 2013;24:1769-77.
19. Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL, Kay E, Mitchell JK, Madi A, Jasani B, James MD, Bridgewater J, Kennedy MJ, Claes B, Lambrechts D, Kaplan R, Cheadle JP; MRC COIN Trial Investigators. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011;377:2103-14.
20. Venook AP, Niedzwiecki D, Lenz H, Innocenti F, Mahoney MR, O'Neil BH, Shaw JE, Polite BN, Hochster HS, Atkins JN, Goldberg RM, Mayer RJ, Schilsky RL, Bertagnolli MM, Blanke CD, Cancer and Leukemia Group B (Alliance), SWOG, and ECOG. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol 2014;32:5s suppl:abstr LBA3.
21. Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Williams R, Rong A, Wiezorek J, Sidhu R, Patterson SD. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023-34.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Zekri J, Farag K, Al-Saadi R, Ashour M, Haggag R. Safety and efficacy of biweekly cetuximab based chemotherapy for patients with metastatic colorectal cancer. J Unexplored Med Data 2016;1:15-20. http://dx.doi.org/10.20517/2572-8180.2016.02
AMA Style
Zekri J, Farag K, Al-Saadi R, Ashour M, Haggag R. Safety and efficacy of biweekly cetuximab based chemotherapy for patients with metastatic colorectal cancer. Journal of Unexplored Medical Data. 2016; 1: 15-20. http://dx.doi.org/10.20517/2572-8180.2016.02
Chicago/Turabian Style
Zekri, Jamal, Kamel Farag, Rawan Al-Saadi, Majed Ashour, Rasha Haggag. 2016. "Safety and efficacy of biweekly cetuximab based chemotherapy for patients with metastatic colorectal cancer" Journal of Unexplored Medical Data. 1: 15-20. http://dx.doi.org/10.20517/2572-8180.2016.02
ACS Style
Zekri, J.; Farag K.; Al-Saadi R.; Ashour M.; Haggag R. Safety and efficacy of biweekly cetuximab based chemotherapy for patients with metastatic colorectal cancer. J. Unexplored. Med. Data. 2016, 1, 15-20. http://dx.doi.org/10.20517/2572-8180.2016.02
About This Article
Copyright
Author Biographies

Data & Comments
Data
 Cite This Article 16 clicks
Cite This Article 16 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.